Page 67 - Book Hosokawa Nanoparticle Technology Handbook
P. 67
FUNDAMENTALS CH. 1 BASIC PROPERTIES AND MEASURING METHODS OF NANOPARTICLES
(5) Corundum structure oxide 100
Many kinds of corundum structure oxide such as Cr O 3
2
and Fe O have existed widely. Cr O and
-Fe O have 90
2
2
3
3
3
2
the same chemical lattice as rhombohedral system, but
Cr O has diamagnetic distribution due to different 80
2
3
spin structure. Especially
-Fe O has small sponta-
2
3
neous magnetization because of the declination of spin Saturation magnetization (Am 2 /kg) 70
interaction.
The magnetization of these substances such 60
-Fe O is so small, and then it is called as parasitic
2
3
ferromagnetism. As the studies of magnetics have 50
been advancing, it has been considered that this is Measured in magnetic field of 796 kA/m
intrinsic magnetism called “weak ferromagnetism” or 40
D-M ferromagnetism [19, 20]. However, Cr O do not 0 30 60 90 120 150
3
2
have weak ferromagnetism. Particle size (nm)
(6) Rutile structure oxide Figure 1.12.1
The relationship between particle size of spherical
Rutile of natural resource has a composition of TiO 2 magnetite and saturation magnetization.
and body-centered cubic structure. Typical magnetic
compounds are MnO and CrO . MnO seems to be
2
2
2
antiferromagnetic substance, however, MnO have
2
a particular spin structure called “screw structure” The decrease in saturation magnetization is not
[21, 22]. Several rare earth metal oxides have the preferable, and the higher characteristics should be
same screw structure. CrO is a peculiar ferromag- maintained in spite of nano-sizing. A lot of studies
2
netic substance whose Cr 4 ions are lined as ferro- have been conducted by the modification of
magnetic distribution. composition or the surface coating onto magnetic
particles in order to find out excellent magnetic
substance which maintains higher magnetic proper-
(7) Magnetoprumbite structure oxide
ties [23, 24].
Magnetic oxides with a hexagonal structure which For example, the oxidation resistance of iron oxide
contains Fe 3 ion, divalent ions M 2 (M Mn, Fe, for magnetic recording media could be improved by
Co, Ni, Cu, Zn, Mg etc.) and other divalent ion such the introduction of surface coating.
2
2
as Ba , Sr , Ca 2 and Pb 2 are called as magneto- Iron-based acicular magnetic metal particles have
prumbite structure magnetic substance. Typical mag- been studied to achieve higher performance of mag-
netic substance is barium ferrite (BaFe O 19 or netic recording. Reducing the size of iron-based metal
12
BaO·6Fe O ). Barium ferrites have large magnetic particles is much required to obtain high signal and
2
3
anisotropy, and can be often applied to permanent low noise of media such as magnetic tapes. The parti-
magnets and magnetic recording materials.
cle size of latest metal particles is about several
decades nanometer, and the size reduction of metal
1.12.4 Magnetic characteristics of nanosized particles would be further continued. Iron-based acic-
materials ular metal particles have high magnetic performance
such as saturation magnetization and coercive force.
The characteristics of nanosized magnetic substance However, it was a problem that they were chemically
are deviated from the bulk ones. Specific surface area unstable and consequently the magnetic properties
becomes larger with decreasing particle size, and then might be deteriorated by surface oxidation for
the influence of surface property becomes serious not instance. The surface coating with carbon was inves-
to be neglected. Generally ferromagnetic substance tigated in order to improve the chemical stability such
would be metals or unstable oxides. Magnetic charac- as oxidation resistance [25].
teristics such as saturation magnetization decrease The timely change in saturation magnetization of
proportionally with size reduction of magnetic magnetic metal particles with the lapse of time was
substance. measured at temperature of 60 C and relative humid-
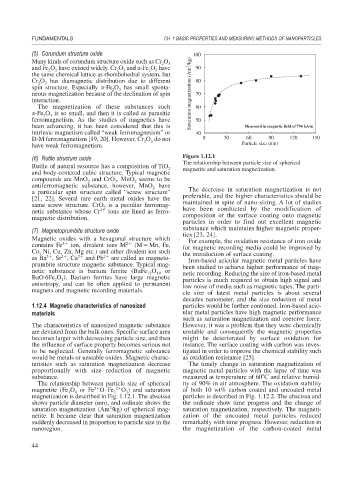
The relationship between particle size of spherical ity of 90% in air atmosphere. The oxidation stability
2
magnetite (Fe O or Fe O·Fe 3 2 O ) and saturation of both 10 wt% carbon coated and uncoated metal
3
3
4
magnetization is described in Fig. 1.12.1. The abscissa particles is described in Fig. 1.12.2. The abscissa and
shows particle diameter (nm), and ordinate shows the the ordinate show time progress and the change of
2
saturation magnetization (Am /kg) of spherical mag- saturation magnetization, respectively. The magneti-
netite. It became clear that saturation magnetization zation of the uncoated metal particles reduced
suddenly decreased in proportion to particle size in the remarkably with time progress. However, reduction in
nanoregion. the magnetization of the carbon-coated metal
44