Page 68 - Book Hosokawa Nanoparticle Technology Handbook
P. 68
1.13 OPTICAL PROPERTY OF NANOPARTICLE FUNDAMENTALS
References
0 10% Carbon-coated [1] S. Chikazumi: Jikken Butsurigaku Kouza 17, Jiki,
Timely change in saturation magnetization (%) -4 Standard iron metal particles [2] S. Chikazumi, S. Miyahara, Y. Ishikawa, M. Asanuma,
-2
iron metal particles
Kyoritsu Shuppan (1968).
N. Wakiyama, Y. Gondo and K. Ohta: Zairyou Kagaku
-6
Kouza 5, Busshitsu no Jikitekiseishitsu, Asakura
Shoten (1968).
-8
humidity of 90% in air atmosphere
Shoukabou (1956).
-10 measured at temperature of 60°C and relative [3] K. Souda, S. Chikazumi: Daigaku Enshu, Denjikigaku,
0 50 100 150 200 250 300 350 [4] S. Chikanobu: Kyoujiseitai no Butsuri, Shoukabou
Exposured time (h) (1959).
[5] S. Chikanobu: Kotai Butsuri 1, 3, 3 (1966).
Figure 1.12.2 [6] H. Miwa, K. Yoshida: Prog. Theoret. Phys., 26, 693
Timely change in saturation magnetization on powder at (1961).
temperature of 60 C and relative humidity of 90% in air [7] T.A. Kaplan: Phys. Rev., 124, 329 (1961).
atmosphere.
[8] R.J. Elliot: Phys. Rev., 124, 340 (1961).
[9] K. Yoshida, A. Watanabe: Prog. Theoret. Phys., 28, 361
(1962).
[10] I. Dzialoshinski: J. Phys. Chem. Solids, 4, 241 (1958).
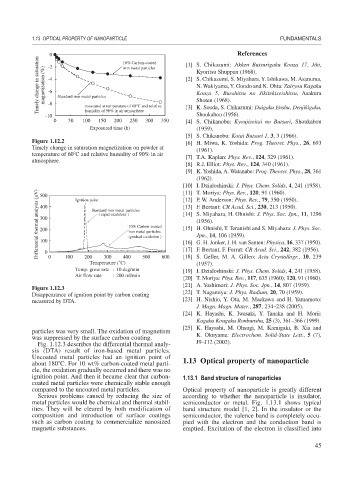
Differential thermal analysis ( V) 400 Standard iron metal particles [13] F. Bertaut: CR Acad. Sci., 230, 213 (1950).
[11] T. Moriya: Phys. Rev., 120, 91 (1960).
500
[12] P. W. Anderson: Phys. Rev., 79, 350 (1950).
Ignition point
[14] S. Miyahara, H. Ohnishi: J. Phys. Soc. Jpn., 11, 1296
( rapid oxidation )
300
(1956).
10% Carbon-coated
[15] H. Ohnishi, T. Teranishi and S. Miyahara: J. Phys. Soc.
200
iron metal particles
Jpn., 14, 106 (1959).
(gradual oxidation )
100
[16] G. H. Jonker, J. H. van Santen: Physica, 16, 337 (1950).
0
300
0
100
[18] S. Geller, M. A. Gilleo: Acta Crystallogr., 10, 239
200
Temperature (˚C) 400 500 600 [17] F. Bertaut, F. Forrat: CR Acad. Sci., 242, 382 (1956).
(1957).
Temp. grow rate : 10 deg/min [19] I. Dzialoshinski: J. Phys. Chem. Solids, 4, 241 (1958).
Air flow rate : 200 ml/min
[20] T. Moriya: Phys. Rev., 117, 635 (1960); 120, 91 (1960).
[21] A. Yoshimori: J. Phys. Soc. Jpn., 14, 807 (1959).
Figure 1.12.3
Disappearance of ignition point by carbon coating [22] T. Nagamiya: J. Phys. Radium, 20, 70 (1959).
measured by DTA. [23] H. Nishio, Y. Ota, M. Maekawa and H. Yamamoto:
J. Magn. Magn. Mater., 287, 234–238 (2005).
[24] K. Hayashi, K. Iwasaki, Y. Tanaka and H. Morii:
Kagaku Kougaku Ronbunshu, 25 (3), 361–366 (1999).
[25] K. Hayashi, M. Ohsugi, M. Kamigaki, B. Xia and
particles was very small. The oxidation of magnetism
was suppressed by the surface carbon coating. K. Okuyama: Electrochem. Solid-State Lett., 5 (7),
Fig. 1.12.3 describes the differential thermal analy- J9–J12 (2002).
sis (DTA) result of iron-based metal particles.
Uncoated metal particles had an ignition point of
about 180 C. For 10 wt% carbon-coated metal parti- 1.13 Optical property of nanoparticle
cle, the oxidation gradually occurred and there was no
ignition point. And then it became clear that carbon- 1.13.1 Band structure of nanoparticles
coated metal particles were chemically stable enough
compared to the uncoated metal particles. Optical property of nanoparticle is greatly different
Serious problems caused by reducing the size of according to whether the nanoparticle is insulator,
metal particles would be chemical and thermal stabil- semiconductor or metal. Fig. 1.13.1 shows typical
ities. They will be cleared by both modification of band structure model [1, 2]. In the insulator or the
composition and introduction of surface coatings semiconductor, the valence band is completely occu-
such as carbon coating to commercialize nanosized pied with the electron and the conduction band is
magnetic substances. emptied. Excitation of the electron is classified into
45