Page 102 - New Trends In Coal Conversion
P. 102
Minimization of Hg and trace elements during coal combustion and gasification processes 65
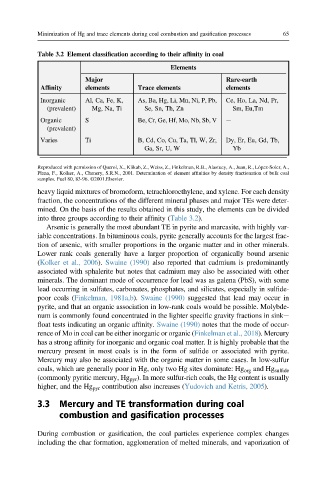
Table 3.2 Element classification according to their affinity in coal
Elements
Major Rare-earth
Affinity elements Trace elements elements
Inorganic Al, Ca, Fe, K, As, Ba, Hg, Li, Mn, Ni, P, Pb, Ce, Ho, La, Nd, Pr,
(prevalent) Mg, Na, Ti Se, Sn, Th, Zn Sm, Eu,Tm
Organic S Be, Cr, Ge, Hf, Mo, Nb, Sb, V e
(prevalent)
Varies Ti B, Cd, Co, Cu, Ta, Tl, W, Zr, Dy, Er, Eu, Gd, Tb,
Ga, Sr, U, W Yb
Reproduced with permission of Querol, X., Klikab, Z., Weiss, Z., Finkelman, R.B., Alastuey, A., Juan, R., L opez-Soler, A.,
Plana, F., Kolker, A., Chenery, S.R.N., 2001. Determination of element affinities by density fractionation of bulk coal
samples. Fuel 80, 83-96. ©2001.Elsevier.
heavy liquid mixtures of bromoform, tetrachloroethylene, and xylene. For each density
fraction, the concentrations of the different mineral phases and major TEs were deter-
mined. On the basis of the results obtained in this study, the elements can be divided
into three groups according to their affinity (Table 3.2).
Arsenic is generally the most abundant TE in pyrite and marcasite, with highly var-
iable concentrations. In bituminous coals, pyrite generally accounts for the largest frac-
tion of arsenic, with smaller proportions in the organic matter and in other minerals.
Lower rank coals generally have a larger proportion of organically bound arsenic
(Kolker et al., 2006). Swaine (1990) also reported that cadmium is predominantly
associated with sphalerite but notes that cadmium may also be associated with other
minerals. The dominant mode of occurrence for lead was as galena (PbS), with some
lead occurring in sulfates, carbonates, phosphates, and silicates, especially in sulfide-
poor coals (Finkelman, 1981a,b). Swaine (1990) suggested that lead may occur in
pyrite, and that an organic association in low-rank coals would be possible. Molybde-
num is commonly found concentrated in the lighter specific gravity fractions in sinke
float tests indicating an organic affinity. Swaine (1990) notes that the mode of occur-
rence of Mo in coal can be either inorganic or organic (Finkelman et al., 2018). Mercury
has a strong affinity for inorganic and organic coal matter. It is highly probable that the
mercury present in most coals is in the form of sulfide or associated with pyrite.
Mercury may also be associated with the organic matter in some cases. In low-sulfur
coals, which are generally poor in Hg, only two Hg sites dominate: Hg org and Hg sulfide
(commonly pyritic mercury, Hg pyr ). In more sulfur-rich coals, the Hg content is usually
higher, and the Hg pyr contribution also increases (Yudovich and Ketris, 2005).
3.3 Mercury and TE transformation during coal
combustion and gasification processes
During combustion or gasification, the coal particles experience complex changes
including the char formation, agglomeration of melted minerals, and vaporization of