Page 107 - New Trends In Coal Conversion
P. 107
70 New Trends in Coal Conversion
100
90
80
Amount of metals retained in different fractions, % 60
70
50
40
30
20
10
0
V Cr Mn Fe Co Ni Cu Zn As Se Cd Sn Sb Hg Ti Pb
Metals
Losses Emissions Fly ash Bottom ash
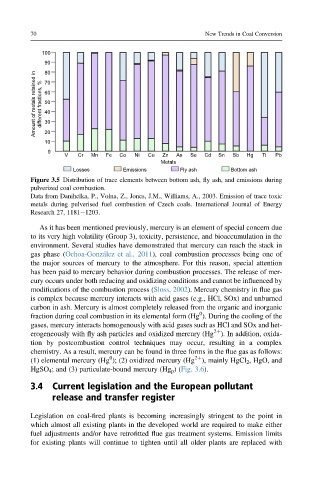
Figure 3.5 Distribution of trace elements between bottom ash, fly ash, and emissions during
pulverized coal combustion.
Data from Danihelka, P., Volna, Z., Jones, J.M., Williams, A., 2003. Emission of trace toxic
metals during pulverised fuel combustion of Czech coals. International Journal of Energy
Research 27, 1181e1203.
As it has been mentioned previously, mercury is an element of special concern due
to its very high volatility (Group 3), toxicity, persistence, and bioaccumulation in the
environment. Several studies have demonstrated that mercury can reach the stack in
gas phase (Ochoa-Gonz alez et al., 2011), coal combustion processes being one of
the major sources of mercury to the atmosphere. For this reason, special attention
has been paid to mercury behavior during combustion processes. The release of mer-
cury occurs under both reducing and oxidizing conditions and cannot be influenced by
modifications of the combustion process (Sloss, 2002). Mercury chemistry in flue gas
is complex because mercury interacts with acid gases (e.g., HCl, SOx) and unburned
carbon in ash. Mercury is almost completely released from the organic and inorganic
0
fraction during coal combustion in its elemental form (Hg ). During the cooling of the
gases, mercury interacts homogenously with acid gases such as HCl and SOx and het-
2þ
erogeneously with fly ash particles and oxidized mercury (Hg ). In addition, oxida-
tion by postcombustion control techniques may occur, resulting in a complex
chemistry. As a result, mercury can be found in three forms in the flue gas as follows:
0
2þ
(1) elemental mercury (Hg ); (2) oxidized mercury (Hg ), mainly HgCl 2 , HgO, and
HgSO 4 ; and (3) particulate-bound mercury (Hg p )(Fig. 3.6).
3.4 Current legislation and the European pollutant
release and transfer register
Legislation on coal-fired plants is becoming increasingly stringent to the point in
which almost all existing plants in the developed world are required to make either
fuel adjustments and/or have retrofitted flue gas treatment systems. Emission limits
for existing plants will continue to tighten until all older plants are replaced with