Page 72 - Numerical methods for chemical engineering
P. 72
58 1 Linear algebra
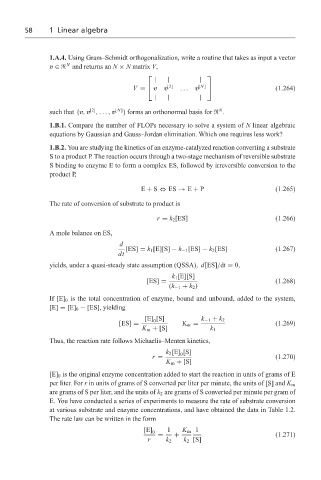
1.A.4. Using Gram–Schmidt orthogonalization, write a routine that takes as input a vector
N
v ∈ and returns an N × N matrix V,
| | |
V = vv [2] ... v [N] (1.264)
| | |
N
[2]
such that {v, v ,..., v [N] } forms an orthonormal basis for .
1.B.1. Compare the number of FLOPs necessary to solve a system of N linear algebraic
equations by Gaussian and Gauss–Jordan elimination. Which one requires less work?
1.B.2. You are studying the kinetics of an enzyme-catalyzed reaction converting a substrate
S to a product P. The reaction occurs through a two-stage mechanism of reversible substrate
S binding to enzyme E to form a complex ES, followed by irreversible conversion to the
product P,
E + S ⇔ ES → E + P (1.265)
The rate of conversion of substrate to product is
r = k 2 [ES] (1.266)
A mole balance on ES,
d
[ES] = k 1 [E][S] − k −1 [ES] − k 2 [ES] (1.267)
dt
yields, under a quasi-steady state assumption (QSSA), d[ES]/dt = 0,
k 1 [E][S]
[ES] = (1.268)
(k −1 + k 2 )
If [E] 0 is the total concentration of enzyme, bound and unbound, added to the system,
[E] = [E] 0 − [ES], yielding
[E] [S] k −1 + k 2
0
[ES] = K m = (1.269)
K m + [S] k 1
Thus, the reaction rate follows Michaelis–Menten kinetics,
k 2 [E] [S]
0
r = (1.270)
K m + [S]
[E] 0 is the original enzyme concentration added to start the reaction in units of grams of E
per liter. For r in units of grams of S converted per liter per minute, the units of [S] and K m
are grams of S per liter, and the units of k 2 are grams of S converted per minute per gram of
E. You have conducted a series of experiments to measure the rate of substrate conversion
at various substrate and enzyme concentrations, and have obtained the data in Table 1.2.
The rate law can be written in the form
[E] 0 1 K m 1
= + (1.271)
r k 2 k 2 [S]