Page 119 - Optical Communications Essentials
P. 119
Photodiodes and Receivers
Photodiodes and Receivers 109
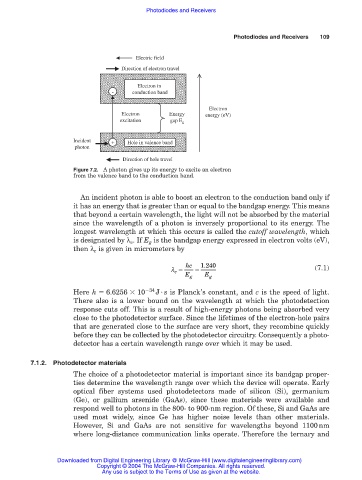
Electric field
Direction of electron travel
Electron in
- conduction band
Electron
Electron Energy energy (eV)
excitation gap E g
Incident + Hole in valence band
photon
Direction of hole travel
Figure 7.2. A photon gives up its energy to excite an electron
from the valence band to the conduction band.
An incident photon is able to boost an electron to the conduction band only if
it has an energy that is greater than or equal to the bandgap energy. This means
that beyond a certain wavelength, the light will not be absorbed by the material
since the wavelength of a photon is inversely proportional to its energy. The
longest wavelength at which this occurs is called the cutoff wavelength, which
is designated by λ c . If E g is the bandgap energy expressed in electron volts (eV),
then λ c is given in micrometers by
hc 1 240
.
λ c (7.1)
E g E g
Here h 6.6256 10 34 J s is Planck’s constant, and c is the speed of light.
There also is a lower bound on the wavelength at which the photodetection
response cuts off. This is a result of high-energy photons being absorbed very
close to the photodetector surface. Since the lifetimes of the electron-hole pairs
that are generated close to the surface are very short, they recombine quickly
before they can be collected by the photodetector circuitry. Consequently a photo-
detector has a certain wavelength range over which it may be used.
7.1.2. Photodetector materials
The choice of a photodetector material is important since its bandgap proper-
ties determine the wavelength range over which the device will operate. Early
optical fiber systems used photodetectors made of silicon (Si), germanium
(Ge), or gallium arsenide (GaAs), since these materials were available and
respond well to photons in the 800- to 900-nm region. Of these, Si and GaAs are
used most widely, since Ge has higher noise levels than other materials.
However, Si and GaAs are not sensitive for wavelengths beyond 1100nm
where long-distance communication links operate. Therefore the ternary and
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.