Page 271 - Optofluidics Fundamentals, Devices, and Applications
P. 271
Optofluidic Dye Lasers 245
1970s, liquid dye has been widely used for frequency tunable and
high-power lasers in the visible frequency range, as reviewed in the
book of Schäfer [6].
The optofluidic dye lasers we are concerned with here employ a
liquid gain medium which consists of organic dye molecules in a suit-
able solvent. The organic dye rhodamine 6G is commonly applied for
lasers emitting in the yellow (vacuum wavelength 570–600 nm), and
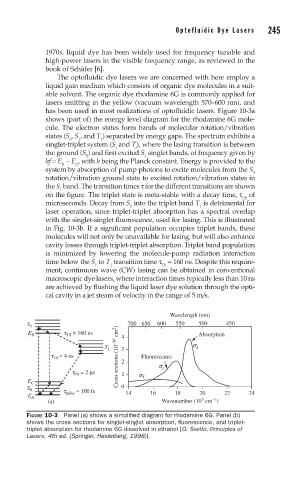
has been used in most realizations of optofluidic lasers. Figure 10-3a
shows (part of) the energy level diagram for the rhodamine 6G mole-
cule. The electron states form bands of molecular rotation/vibration
states (S , S , and T ) separated by energy gaps. The spectrum exhibits a
0 1 1
singlet-triplet system (S and T), where the lasing transition is between
i i
the ground (S ) and first excited S singlet bands, of frequency given by
0 1
hf = E − E , with h being the Planck constant. Energy is provided to the
B C
system by absorption of pump photons to excite molecules from the S
0
rotation/vibration ground state to excited rotation/vibration states in
the S band. The transition times τ for the different transitions are shown
1
on the figure. The triplet state is meta-stable with a decay time, τ of
Ph
microseconds. Decay from S into the triplet band T is detrimental for
1 1
laser operation, since triplet-triplet absorption has a spectral overlap
with the singlet-singlet fluorescence, used for lasing. This is illustrated
in Fig. 10-3b. If a significant population occupies triplet bands, these
molecules will not only be unavailable for lasing, but will also enhance
cavity losses through triplet-triplet absorption. Triplet band population
is minimized by lowering the molecule-pump radiation interaction
time below the S to T transition time τ = 160 ns. Despite this require-
1 1 TS
ment, continuous wave (CW) lasing can be obtained in conventional
macroscopic dye lasers, where interaction times typically less than 10 ns
are achieved by flushing the liquid laser dye solution through the opti-
cal cavity in a jet steam of velocity in the range of 5 m/s.
Wavelength (nm)
700 650 600 550 500 450
S 1
Cross sections (10 –16 cm 2 ) 2
t = 160 ns
E B TS 4 Absorption
T 1 3 s a
t ≈ 4 ns Fluorescence
10
= 2 μs s e
t Ph 1 s T
E C
S 0 t ≈ 100 fs 0
E A relax 14 16 18 20 22 24
3
–1
(a) Wavenumber (10 cm )
FIGURE 10-3 Panel (a) shows a simplified diagram for rhodamine 6G. Panel (b)
shows the cross sections for singlet-singlet absorption, fluorescence, and triplet-
triplet absorption for rhodamine 6G dissolved in ethanol [O. Svelto, Principles of
Lasers, 4th ed. (Springer, Heidelberg, 1998)].