Page 228 - Organic Electronics in Sensors and Biotechnology
P. 228
An Intr oduction to Or ganic Photodetectors 205
O O
MeO MeO
(e) (f)
(a)
OCH 3
O
(b)
(g)
S S
* S S *
n
(c)
(h)
(d)
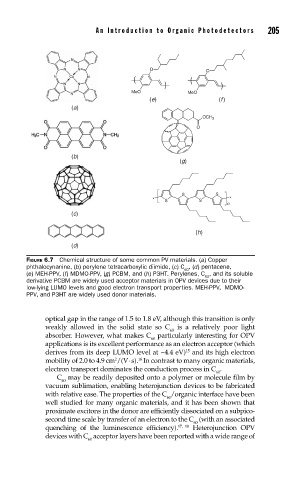
FIGURE 6.7 Chemical structure of some common PV materials. (a) Copper
phthalocynanine, (b) perylene tetracarboxylic diimide, (c) C , (d) pentacene,
60
(e) MEH-PPV, (f) MDMO-PPV, (g) PCBM, and (h) P3HT. Perylenes, C , and its soluble
60
derivative PCBM are widely used acceptor materials in OPV devices due to their
low-lying LUMO levels and good electron transport properties. MEH-PPV, MDMO-
PPV, and P3HT are widely used donor materials.
optical gap in the range of 1.5 to 1.8 eV, although this transition is only
weakly allowed in the solid state so C is a relatively poor light
60
absorber. However, what makes C particularly interesting for OPV
60
applications is its excellent performance as an electron acceptor (which
15
derives from its deep LUMO level at ~4.4 eV) and its high electron
2
16
mobility of 2.0 to 4.9 cm /(V . s). In contrast to many organic materials,
electron transport dominates the conduction process in C .
60
C may be readily deposited onto a polymer or molecule film by
60
vacuum sublimation, enabling heterojunction devices to be fabricated
with relative ease. The properties of the C /organic interface have been
60
well studied for many organic materials, and it has been shown that
proximate excitons in the donor are efficiently dissociated on a subpico-
second time scale by transfer of an electron to the C (with an associated
60
quenching of the luminescence efficiency). 17, 18 Heterojunction OPV
devices with C acceptor layers have been reported with a wide range of
60