Page 360 - Organic Electronics in Sensors and Biotechnology
P. 360
Luminescent Conjugated Polymers for Staining and Characterization of Amyloid Deposits 337
processes is carried out by using the conformational changes of cat-
ionic, anionic, and zwitter-ionic polythiophene derivatives, which are
noncovalently attached to the biomolecule of interest.
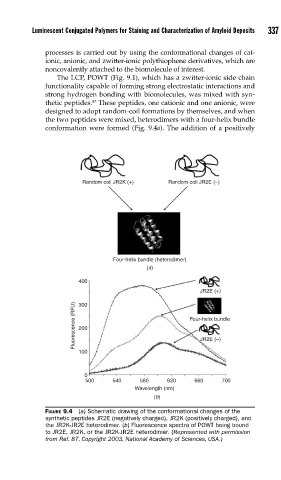
The LCP, POWT (Fig. 9.1), which has a zwitter-ionic side chain
functionality capable of forming strong electrostatic interactions and
strong hydrogen bonding with biomolecules, was mixed with syn-
87
thetic peptides. These peptides, one cationic and one anionic, were
designed to adopt random-coil formations by themselves, and when
the two peptides were mixed, heterodimers with a four-helix bundle
conformation were formed (Fig. 9.4a). The addition of a positively
Random coil JR2K (+) Random coil JR2E (–)
Four-helix bundle (heterodimer)
(a)
400
JR2E (+)
Fluorescence (RFU) 200 Four-helix bundle
300
100 JR2E (–)
0
500 540 580 620 660 700
Wavelength (nm)
(b)
FIGURE 9.4 (a) Schematic drawing of the conformational changes of the
synthetic peptides JR2E (negatively charged), JR2K (positively charged), and
the JR2K-JR2E heterodimer. (b) Fluorescence spectra of POWT being bound
to JR2E, JR2K, or the JR2K-JR2E heterodimer. (Represented with permission
from Ref. 87. Copyright 2003, National Academy of Sciences, USA.)