Page 365 - Organic Electronics in Sensors and Biotechnology
P. 365
342 Chapter Nine
appropriate when fibrils morphologically and structurally related to
extracellular amyloid form inside the cell. 95
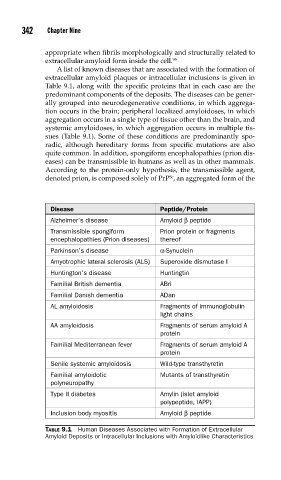
A list of known diseases that are associated with the formation of
extracellular amyloid plaques or intracellular inclusions is given in
Table 9.1, along with the specific proteins that in each case are the
predominant components of the deposits. The diseases can be gener-
ally grouped into neurodegenerative conditions, in which aggrega-
tion occurs in the brain; peripheral localized amyloidoses, in which
aggregation occurs in a single type of tissue other than the brain, and
systemic amyloidoses, in which aggregation occurs in multiple tis-
sues (Table 9.1). Some of these conditions are predominantly spo-
radic, although hereditary forms from specific mutations are also
quite common. In addition, spongiform encephalopathies (prion dis-
eases) can be transmissible in humans as well as in other mammals.
According to the protein-only hypothesis, the transmissible agent,
denoted prion, is composed solely of PrP , an aggregated form of the
Sc
Disease Peptide/Protein
Alzheimer’s disease Amyloid β peptide
Transmissible spongiform Prion protein or fragments
encephalopathies (Prion diseases) thereof
Parkinson’s disease α-Synuclein
Amyotrophic lateral sclerosis (ALS) Superoxide dismutase I
Huntington’s disease Huntingtin
Familial British dementia ABri
Familial Danish dementia ADan
AL amyloidosis Fragments of immunoglobulin
light chains
AA amyloidosis Fragments of serum amyloid A
protein
Familial Mediterranean fever Fragments of serum amyloid A
protein
Senile systemic amyloidosis Wild-type transthyretin
Familial amyloidotic Mutants of transthyretin
polyneuropathy
Type II diabetes Amylin (islet amyloid
polypeptide, IAPP)
Inclusion body myositis Amyloid β peptide
TABLE 9.1 Human Diseases Associated with Formation of Extracellular
Amyloid Deposits or Intracellular Inclusions with Amyloidlike Characteristics