Page 370 - Organic Electronics in Sensors and Biotechnology
P. 370
Luminescent Conjugated Polymers for Staining and Characterization of Amyloid Deposits 347
backbone. Additionally, the chain length distribution of these materials
was shown to be rather narrow, and around 90% of the material had a
well-defined chain length of 9 or 12 monomers, although they were syn-
14
thesized by random polymerization. Both of these properties––the well
defined chain length and the enhancement of the conformational
freedom––were shown to improve the specificity for amyloid fibrils
and the spectral assignment of distinct protein conformations.
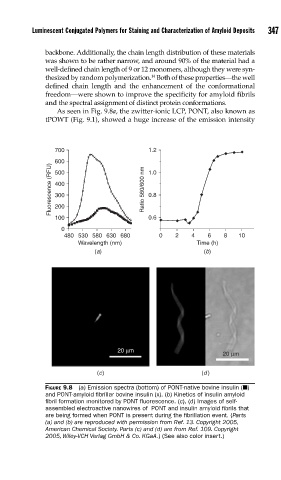
As seen in Fig. 9.8a, the zwitter-ionic LCP, PONT, also known as
tPOWT (Fig. 9.1), showed a huge increase of the emission intensity
700 1.2
Fluorescence (RFU) 600 Ratio 560/600 nm 1.0
500
400
0.8
300
200
100 0.6
0
480 530 580 630 680 0 2 4 6 8 10
Wavelength (nm) Time (h)
(a) (b)
20 μm
20 μm
(c) (d )
FIGURE 9.8 (a) Emission spectra (bottom) of PONT-native bovine insulin (Q)
and PONT-amyloid fi brillar bovine insulin (x). (b) Kinetics of insulin amyloid
fi bril formation monitored by PONT fl uorescence. (c), (d) Images of self-
assembled electroactive nanowires of PONT and insulin amyloid fi brils that
are being formed when PONT is present during the fi brillation event. (Parts
(a) and (b) are reproduced with permission from Ref. 13. Copyright 2005,
American Chemical Society. Parts (c) and (d) are from Ref. 109. Copyright
2005, Wiley-VCH Verlag GmbH & Co. KGaA.) (See also color insert.)