Page 304 - Origin and Prediction of Abnormal Formation Pressures
P. 304
274 H.H. RIEKE, G.V. CHILINGAR AND J.O. ROBERTSON JR.
i>//. '/9
100
//
75 '
// .o_
.I_" 50
12I
0
25
/ /4: ..>
;. ;;.,"..:..;
. . . . . 100
|
0 5 10 15 20 25
Compaction Time, days
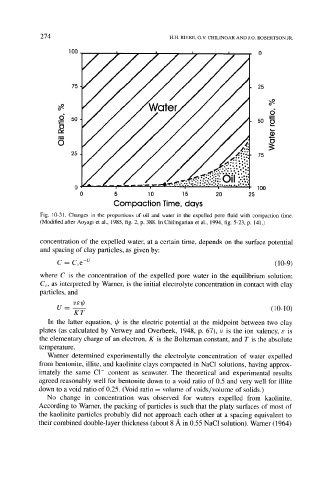
Fig. 10-31. Changes in the proportions of oil and water in the expelled pore fluid with compaction time.
(Modified after Aoyagi et al., 1985, fig. 2, p. 388. In Chilingarian et al., 1994, fig. 5-23, p. 141.)
concentration of the expelled water, at a certain time, depends on the surface potential
and spacing of clay particles, as given by:
C - Coe -U (I0-9)
where C is the concentration of the expelled pore water in the equilibrium solution;
Co, as interpreted by Warner, is the initial electrolyte concentration in contact with clay
particles, and
vs~
U -- (10-10)
KT
In the latter equation, ~ is the electric potential at the midpoint between two clay
plates (as calculated by Verwey and Overbeek, 1948, p. 67), v is the ion valency, s is
the elementary charge of an electron, K is the Boltzman constant, and T is the absolute
temperature.
Warner determined experimentally the electrolyte concentration of water expelled
from bentonite, illite, and kaolinite clays compacted in NaCI solutions, having approx-
imately the same C1- content as seawater. The theoretical and experimental results
agreed reasonably well for bentonite down to a void ratio of 0.5 and very well for illite
down to a void ratio of 0.25. (Void ratio = volume of voids/volume of solids.)
No change in concentration was observed for waters expelled from kaolinite.
According to Warner, the packing of particles is such that the platy surfaces of most of
the kaolinite particles probably did not approach each other at a spacing equivalent to
their combined double-layer thickness (about 8 ,& in 0.55 NaC1 solution). Warner (1964)