Page 303 - Origin and Prediction of Abnormal Formation Pressures
P. 303
PORE WATER COMPACTION CHEMISTRY AS RELATED TO OVERPRESSURES 273
Temperature, ~
200 220 240 260 280 300 320 340
0 I I I I ~ [ I I " I I I I I
13AI,
5,000
\
.m
D. I0,000
r
t~
15,000
\
\ I
20,000 \ I
\
\
\
25,000 \
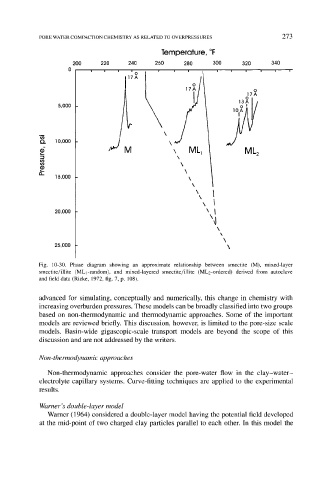
Fig. 10-30. Phase diagram showing an approximate relationship between smectite (M), mixed-layer
smectite/illite (MLj-random), and mixed-layered smectite/illite (MLz-ordered) derived from autoclave
and field data (Rieke, 1972, fig. 7, p. 108).
advanced for simulating, conceptually and numerically, this change in chemistry with
increasing overburden pressures. These models can be broadly classified into two groups
based on non-thermodynamic and thermodynamic approaches. Some of the important
models are reviewed briefly. This discussion, however, is limited to the pore-size scale
models. Basin-wide gigascopic-scale transport models are beyond the scope of this
discussion and are not addressed by the writers.
Non-thermodynamic approaches
Non-thermodynamic approaches consider the pore-water flow in the clay-water-
electrolyte capillary systems. Curve-fitting techniques are applied to the experimental
results.
Warner's double-layer model
Warner (1964) considered a double-layer model having the potential field developed
at the mid-point of two charged clay particles parallel to each other. In this model the