Page 298 - Origin and Prediction of Abnormal Formation Pressures
P. 298
PORE WATER COMPACTION CHEMISTRY AS RELATED TO OVERPRESSURES 269
i . . . . . . ! 9 9 9 ' 9 "I ' ' ' ' . . .i i --
130
C 120
0
..== : : : 9 -: : : : : :- ~ . .~ 9 .-
: : : : :- - : 9 : :: : . : : ::
a ~ ~ i ~ ~i~i~ . . . . . .
...- .i..i-,.-~. ............................... i..i .i-!..;.!-
110 ................................... i..:-~.~.!.i .............................
- i iiii! ! i~ii~ ~ ~~i
C
fl) ........................... i 9 " ~'" i ~ i ~ ~i _..*k---,i....'."i :. " -:-"
" " ~ . - . ~ . ' . : ; - t ~ ~ ~ . "
. ."*"'i " ""..i..~..i..~.'._
0 100
C .===
0 ~
.;.
i.
. ......
o O. 90 ..... Na + " .~..L.i..;.i.~ ............ ~ ....... ~..." .~ .... ;..i..i.,.~ ............ ~ ....... ; ..... i .... ;..;..i..~..i.;..-"
--o - _._K + i i i i ~ -
:
._a : ...... : : :: ., i i . . ~ ~ i i i ii : i ~ :. ! :: ii -
:
.
.
:
.
.-
.
.
: :
.
.
.
.
.
"
*
" ~ ~ ~"
80 . "--!--...-,.-.-.-Ca ~+ ~~~~~~ ............ ~-"~,"~ " .... ~ " ~ ~ ? ~ ? ~ ............................... ~-~"~.i"i..- "
~
~ " ~ :
:
:
" :
.,. Mg 2+ ! i ! ~ii ,,i ~ ~ ! ! ~i :: i i !~i -
.==-
70 ' .............. i i iii! ! ~. i !i~!~ ~ ~ ~ -
O . . . . S O 4 2- .~ 9 --:-- -.- -.~ -.~. ! ............ .: ....... .~ .... .. ..~---.- 9 + 9 ~.- :. ! ............ ! ....... ~ ..... .~ 9 - -'.: - 9 i 9 - ! 9 -!- -.: -:.- - .-
i
i
i
!"-!
i
i
~ ~
i
~ ! !i
i!
i
~ !
~
!
~
i
60 -~- Cl i i iill i i i i i!~! -.k i i i ! ;iil
.................. "-"':":":"~'~ ............................. ":"~ '"':"-~ .... -'"'~"'~"~ ..... i .... ~"i"i i"~*i"-
o --..- TDS i i iili i !ii!i * ~ , ~ "
~ i i iii i i ~ iii i ::,,:: i ! :: i~
r~ 5 0 "~ ............. ~ ....... ".: ..... i-!---.:--.:---~~ ............ ~ ....... :- .... !-..-.:.--.:---i-.i-.~--~ ............ ,~ ....... i ..... !-..~-..!.i.!-.:-.;..-
i i i iiiiii! i i i!iiill i !iiii!ii
4 0 --:- ............. :. ....... :- ..... ......i..-:..-..-.!.! ............ ! ....... - .... ~...~...:....i..~..i...: .............. ! ....... ! ..... ! .... !...i..i.!..;.;..,
:, i i i i :,i~i i ~ i i i i ill i : i i i i i:i ,
10 1 O0 1000 1 O, 000
Axial pressure, psi
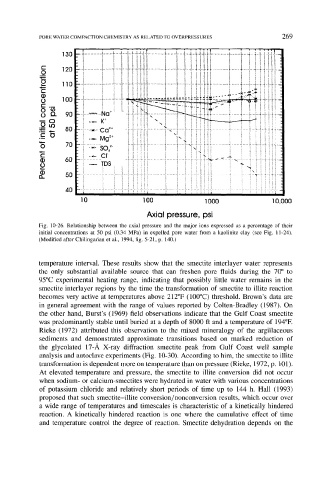
Fig. 10-26. Relationship between the axial pressure and the major ions expressed as a percentage of their
initial concentrations at 50 psi (0.34 MPa) in expelled pore water from a kaolinite clay (see Fig. 11-24).
(Modified after Chilingarian et al., 1994, fig. 5-21, p. 140.)
temperature interval. These results show that the smectite interlayer water represents
the only substantial available source that can freshen pore fluids during the 70 ~ to
95~ experimental heating range, indicating that possibly little water remains in the
smectite interlayer regions by the time the transformation of smectite to illite reaction
becomes very active at temperatures above 212~ (100~ threshold. Brown's data are
in general agreement with the range of values reported by Colten-Bradley (1987). On
the other hand, Burst's (1969) field observations indicate that the Gulf Coast smectite
was predominantly stable until buried at a depth of 8000 ft and a temperature of 194~
Rieke (1972) attributed this observation to the mixed mineralogy of the argillaceous
sediments and demonstrated approximate transitions based on marked reduction of
the glycolated 17-A X-ray diffraction smectite peak from Gulf Coast well sample
analysis and autoclave experiments (Fig. 10-30). According to him, the smectite to illite
transformation is dependent more on temperature than on pressure (Rieke, 1972, p. 101).
At elevated temperature and pressure, the smectite to illite conversion did not occur
when sodium- or calcium-smectites were hydrated in water with various concentrations
of potassium chloride and relatively short periods of time up to 144 h. Hall (1993)
proposed that such smectite-illite conversion/nonconversion results, which occur over
a wide range of temperatures and timescales is characteristic of a kinetically hindered
reaction. A kinetically hindered reaction is one where the cumulative effect of time
and temperature control the degree of reaction. Smectite dehydration depends on the