Page 296 - Origin and Prediction of Abnormal Formation Pressures
P. 296
PORE WATER COMPACTION CHEMISTRY AS RELATED TO OVERPRESSURES 267
/
_ .: t n"n~ + l
0 -- "1" K + .
0
0 " ,I, -- ~a 2+ "
4 ...................................................... .~ .................................................................. -._
0 . = Mg 2+
X _ * SO. ~- .
- i m . c'i -
,~,o
"',~,~
E 3 ....................... - .......... :" ........................................................................ i
.
.
.
- 9 .. " 9
0 i
=.,..
=in=
2 ............................. ".-" .................................................. .'}'" ....................................................
c
- " x x : ; : i " "
0 _ x• • ! " -
c
0 1 -i i- f "
o " ............................................................ : "~• ......... " ......................................................
x
x
i
-- : : X x "
i -:"
-- ,.,
i .:
o _..)...a ................ i. ,i $ii ~~i).i...i../~ i.i. ~)~ ~ ~ . ....... ~ ......................... ._
i
t
i
i
"
1 l 0 l O0 1000 10,000 100,000
Axial pressure, psi
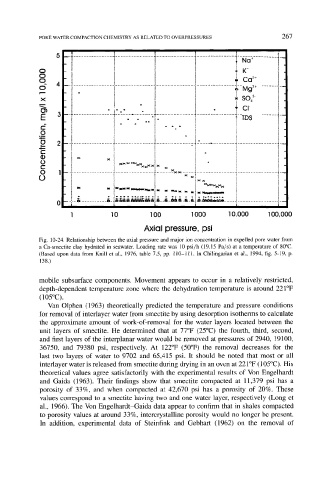
Fig. 10-24. Relationship between the axial pressure and major ion concentration in expelled pore water from
a Ca-smectite clay hydrated in seawater. Loading rate was 10 psi/h (19.15 Pa/s) at a temperature of 80~
(Based upon data from Knill et al., 1976, table 7.5, pp. 110-111. In Chilingarian et al., 1994, fig. 5-19, p.
138.)
mobile subsurface components. Movement appears to occur in a relatively restricted,
depth-dependent temperature zone where the dehydration temperature is around 221~
(]05oc).
Van Olphen (1963) theoretically predicted the temperature and pressure conditions
for removal of interlayer water from smectite by using desorption isotherms to calculate
the approximate amount of work-of-removal for the water layers located between the
unit layers of smectite. He determined that at 77~ (25~ the fourth, third, second,
and first layers of the interplanar water would be removed at pressures of 2940, 19100,
36750, and 79380 psi, respectively. At 122~ (50~ the removal decreases for the
last two layers of water to 9702 and 65,415 psi. It should be noted that most or all
interlayer water is released from smectite during drying in an oven at 221 ~ (105~ His
theoretical values agree satisfactorily with the experimental results of Von Engelhardt
and Gaida (1963). Their findings show that smectite compacted at 11,379 psi has a
porosity of 33%, and when compacted at 42,670 psi has a porosity of 20%. These
values correspond to a smectite having two and one water layer, respectively (Long et
al., 1966). The Von Engelhardt-Gaida data appear to confirm that in shales compacted
to porosity values at around 33%, intercrystalline porosity would no longer be present.
In addition, experimental data of Steinfink and Gebhart (1962) on the removal of