Page 293 - Origin and Prediction of Abnormal Formation Pressures
P. 293
264 H.H. RIEKE, G.V. CHILINGAR AND J.O. ROBERTSON JR.
! .... ! l
.......................... "t ........................ t ......................... | .................................................. ~'-
~ ~ ! ' Na + "
.::-".-'"-"-~'...-. ~i " '"
o K +
o "i" : o
(:3 ...~ ......................... - ......................... ) .......................... } .... ".~., ..................... C&+
i '
'
|
"
w,
9
t
d 4 ........ ".'...,,,,
i : : " Mg ~+
r---
X i } | R ~ SO4 ~-
.
| 9 9
13) i i i i ..~. '~ Cl-
E 3 --~: ........................ + ........................ ......... ..,..
9
i
i , ! ................................................... TDS
9 i
O { 9 i
-i.-- " , i -!
.................... .....~..~....--...o..~.~.,.'-t....o....o....,...,,o,,.~ ..................................................
i o, ... 9 ~4,o
e--
- i .: i
0
c-
O 1 g~ ................. 9 --~ .... ~,.-~-v;-~-;...;-~., .... ~"'";"'L .......... | ........................... .,,..
0 . i - "'"i'.. "
- i ~ i i "--..,,... .i
i i i i -
i" " ~" --"------- ~ ~ ~ mot,~,,,,,,,,,m~ ! i -
~...!.~. ""= *.~~.
...... ............... ,,* ~...~,.. ~...~. ~ ~ ~ ..... ......................... ~..,J
4 0 ~ 0
l l 0 l O0 1000 l 0,000 100,000
Axial pressure, psi
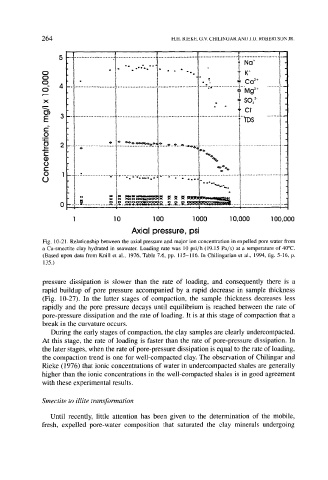
Fig. 10-21. Relationship between the axial pressure and major ion concentration in expelled pore water from
a Ca-smectite clay hydrated in seawater. Loading rate was 10 psi/h (19.15 Pa/s) at a temperature of 40~
(Based upon data from Knill et al., 1976, Table 7.6, pp. 115-116. In Chilingarian et al., 1994, fig. 5-16, p.
135.)
pressure dissipation is slower than the rate of loading, and consequently there is a
rapid buildup of pore pressure accompanied by a rapid decrease in sample thickness
(Fig. 10-27). In the latter stages of compaction, the sample thickness decreases less
rapidly and the pore pressure decays until equilibrium is reached between the rate of
pore-pressure dissipation and the rate of loading. It is at this stage of compaction that a
break in the curvature occurs.
During the early stages of compaction, the clay samples are clearly undercompacted.
At this stage, the rate of loading is faster than the rate of pore-pressure dissipation. In
the later stages, when the rate of pore-pressure dissipation is equal to the rate of loading,
the compaction trend is one for well-compacted clay. The observation of Chilingar and
Rieke (1976) that ionic concentrations of water in undercompacted shales are generally
higher than the ionic concentrations in the well-compacted shales is in good agreement
with these experimental results.
Smectite to illite transformation
Until recently, little attention has been given to the determination of the mobile,
fresh, expelled pore-water composition that saturated the clay minerals undergoing