Page 290 - Origin and Prediction of Abnormal Formation Pressures
P. 290
PORE WATER COMPACTION CHEMISTRY AS RELATED TO OVERPRESSURES 261
~E E 600 Ca ++
0
e-. .
._o ~
"-8
"" (-. 500
o ~
e-.
0
0 400
0 20 40 60 80 l O0
Cumulative volume of
expelled solution, ml
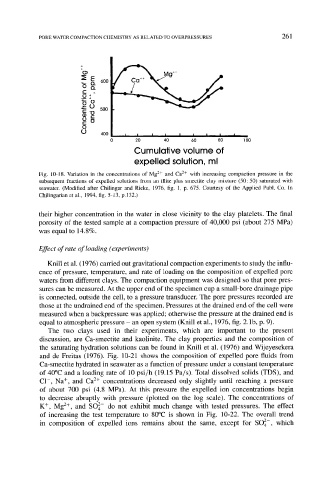
Fig. 10-18. Variation in the concentrations of Mg 2+ and Ca 2+ with increasing compaction pressure in the
subsequent fractions of expelled solutions from an illite plus smectite clay mixture (50"50) saturated with
seawater. (Modified after Chilingar and Rieke, 1976, fig. 1, p. 675. Courtesy of the Applied Publ. Co. In
Chilingarian et al., 1994, fig. 5-13, p.132.)
their higher concentration in the water in close vicinity to the clay platelets. The final
porosity of the tested sample at a compaction pressure of 40,000 psi (about 275 MPa)
was equal to 14.8%.
Effect of rate of loading (experiments)
Knill et al. (1976) carried out gravitational compaction experiments to study the influ-
ence of pressure, temperature, and rate of loading on the composition of expelled pore
waters from different clays. The compaction equipment was designed so that pore pres-
sures can be measured. At the upper end of the specimen cup a small-bore drainage pipe
is connected, outside the cell, to a pressure transducer. The pore pressures recorded are
those at the undrained end of the specimen. Pressures at the drained end of the cell were
measured when a backpressure was applied; otherwise the pressure at the drained end is
equal to atmospheric pressure - an open system (Knill et al., 1976, fig. 2.1b, p. 9).
The two clays used in their experiments, which are important to the present
discussion, are Ca-smectite and kaolinite. The clay properties and the composition of
the saturating hydration solutions can be found in Knill et al. (1976) and Wijeyesekera
and de Freitas (1976). Fig. 10-21 shows the composition of expelled pore fluids from
Ca-smectite hydrated in seawater as a function of pressure under a constant temperature
of 40~ and a loading rate of 10 psi/h (19.15 Pa/s). Total dissolved solids (TDS), and
CI-, Na +, and Ca 2+ concentrations decreased only slightly until reaching a pressure
of about 700 psi (4.8 MPa). At this pressure the expelled ion concentrations begin
to decrease abruptly with pressure (plotted on the log scale). The concentrations of
K +, Mg 2+, and SO 2- do not exhibit much change with tested pressures. The effect
of increasing the test temperature to 80~ is shown in Fig. 10-22. The overall trend
in composition of expelled ions remains about the same, except for SO]-, which