Page 291 - Origin and Prediction of Abnormal Formation Pressures
P. 291
262 H.H. RIEKE, G.V. CHILINGAR AND J.O. ROBERTSON JR.
20,000 r
E
O.
O.
0
0
IC
0 10,000
13
(!)
0
IC
0
s
0
0 20 40 60 80 100
Cumulative volume
of expelled solution, ml
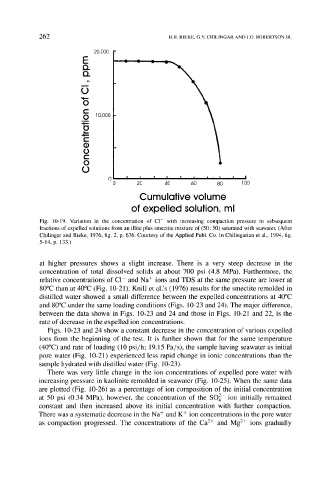
Fig. 10-19. Variation in the concentration of Cl- with increasing compaction pressure in subsequent
fractions of expelled solutions from an illite plus smectite mixture of (50: 50) saturated with seawater. (After
Chilingar and Rieke, 1976, fig. 2, p. 676. Courtesy of the Applied Publ. Co. In Chilingarian et al., 1994, fig.
5-14, p. 133.)
at higher pressures shows a slight increase. There is a very steep decrease in the
concentration of total dissolved solids at about 700 psi (4.8 MPa). Furthermore, the
relative concentrations of C1- and Na + ions and TDS at the same pressure are lower at
80~ than at 40~ (Fig. 10-21). Knill et al.'s (1976) results for the smectite remolded in
distilled water showed a small difference between the expelled concentrations at 40~
and 80~ under the same loading conditions (Figs. 10-23 and 24). The major difference,
between the data shown in Figs. 10-23 and 24 and those in Figs. 10-21 and 22, is the
rate of decrease in the expelled ion concentrations.
Figs. 10-23 and 24 show a constant decrease in the concentration of various expelled
ions from the beginning of the test. It is further shown that for the same temperature
(40~ and rate of loading (10 psi/h; 19.15 Pa/s), the sample having seawater as initial
pore water (Fig. 10-21) experienced less rapid change in ionic concentrations than the
sample hydrated with distilled water (Fig. 10-23).
There was very little change in the ion concentrations of expelled pore water with
increasing pressure in kaolinite remolded in seawater (Fig. 10-25). When the same data
are plotted (Fig. 10-26) as a percentage of ion composition of the initial concentration
at 50 psi (0.34 MPa), however, the concentration of the SO 2- ion initially remained
constant and then increased above its initial concentration with further compaction.
There was a systematic decrease in the Na + and K + ion concentrations in the pore water
as compaction progressed. The concentrations of the Ca 2+ and Mg 2+ ions gradually