Page 294 - Origin and Prediction of Abnormal Formation Pressures
P. 294
PORE WATER COMPACTION CHEMISTRY AS RELATED TO OVERPRESSURES 265
_." ........................ ; ........................ :..- ......................... : ......................... i ...........................
!
- i, i ) .*Na + -
~
0 i ) "K+
0 i " i - ii . t~,-, 2+ "
0 - i . . i .! . . 9 ,..,., -
d 4 "i ........................ +---a,- .... -'- --'- 9 -'-
+i t ..: .. ? Mg 2+ ...............
,- § ~ "
X ,i, S04~-
- i + +
i
- | i * cr
13) 3 . . . . . . . . . . . . $ . .-m,
E .......................... + ........................ + ........................... ".. TDS ~
ff l
0
13 2 o "go ~ .:g-~..~,.~.w.;;.g~ ........ g...g--.w ................. ~. ........................................
!,._..
c" " i i .: ~ ~o o "
" i :" : o o " "
- ) ) + "~~ " "~ "
0 - ~ : . =: ~ .
1 --..~ ........................ :.g ........................ ~- ......................... : ......................... ~ ......................... .i-.--
0 -. + ....... ~ - - - - -i. )
o
- II1 X X
,
0 "T ...... ~' ...... m.~. ..... .~...m..~...,i,.~.:.~..~;i i .... ~"~'~""~"~'~~~i ...................................
l l 0 l O0 1000 l 0,000 100,000
Axial pressure, psi
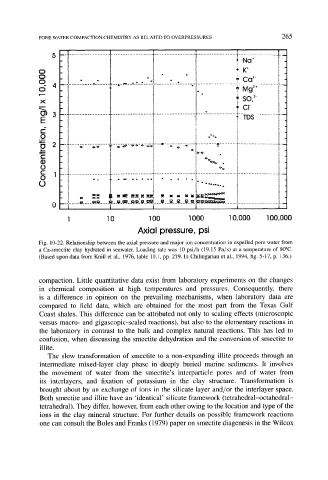
Fig. 10-22. Relationship between the axial pressure and major ion concentration in expelled pore water from
a Ca-smectite clay hydrated in seawater. Loading rate was 10 psi/h (19.15 Pa/s) at a temperature of 80~
(Based upon data from Knill et al., 1976, table 10.1, pp. 219. In Chilingarian et al., 1994, fig. 5-17, p. 136.)
compaction. Little quantitative data exist from laboratory experiments on the changes
in chemical composition at high temperatures and pressures. Consequently, there
is a difference in opinion on the prevailing mechanisms, when laboratory data are
compared to field data, which are obtained for the most part from the Texas Gulf
Coast shales. This difference can be attributed not only to scaling effects (microscopic
versus macro- and gigascopic-scaled reactions), but also to the elementary reactions in
the laboratory in contrast to the bulk and complex natural reactions. This has led to
confusion, when discussing the smectite dehydration and the conversion of smectite to
illite.
The slow transformation of smectite to a non-expanding illite proceeds through an
intermediate mixed-layer clay phase in deeply buried marine sediments. It involves
the movement of water from the smectite's interparticle pores and of water from
its interlayers, and fixation of potassium in the clay structure. Transformation is
brought about by an exchange of ions in the silicate layer and/or the interlayer space.
Both smectite and illite have an 'identical' silicate framework (tetrahedral-octahedral-
tetrahedral). They differ, however, from each other owing to the location and type of the
ions in the clay mineral structure. For further details on possible framework reactions
one can consult the Boles and Franks (1979) paper on smectite diagenesis in the Wilcox