Page 289 - Origin and Prediction of Abnormal Formation Pressures
P. 289
260 H.H. RIEKE, G.V. CHILINGAR AND J.O. ROBERTSON JR.
A (cations) B (anions)
F HC03CI S02 I K + Ca 2+ Mg2+Na§
50 .00 950016d00 I00 '00 ~00 ~k ' " ,
-\
E - \ /
C~ Na+ i
c)
m.
m O0 300 9000
12) 300 ~00 700
E
C:
0 \
m~,,
0 , co3- \
Imm 9 ~/, "
C: 50 20C 1850( 200 500 600
0
0 9 I:
r"
0 / \
..... W- ..... ..',,,t#
.
, , I
wO -10C "1800 I , t ~.. "10C "40C "500
0 20 40 (~0 20 40 60
Amount of Extruded Solution, cm 3
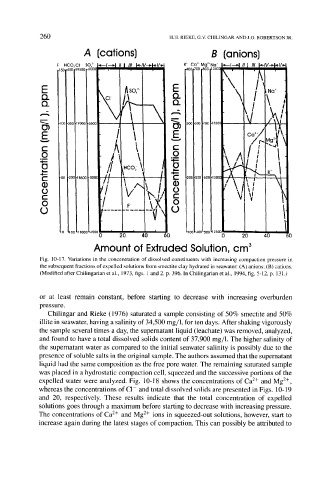
Fig. 10-17. Variations in the concentration of dissolved constituents with increasing compaction pressure in
the subsequent fractions of expelled solutions from smectite clay hydrated in seawater: (A) anions; (B) cations.
(Modified after Chilingarian et al., 1973, figs. 1 and 2, p. 396. In Chilingarian et al., 1994, fig. 5-12, p. 131.)
or at least remain constant, before starting to decrease with increasing overburden
pressure.
Chilingar and Rieke (1976) saturated a sample consisting of 50% smectite and 50%
illite in seawater, having a salinity of 34,500 mg/1, for ten days. After shaking vigorously
the sample several times a day, the supernatant liquid (leachate) was removed, analyzed,
and found to have a total dissolved solids content of 37,900 mg/1. The higher salinity of
the supernatant water as compared to the initial seawater salinity is possibly due to the
presence of soluble salts in the original sample. The authors assumed that the supernatant
liquid had the same composition as the free pore water. The remaining saturated sample
was placed in a hydrostatic compaction cell, squeezed and the successive portions of the
expelled water were analyzed. Fig. 10-18 shows the concentrations of Ca 2+ and Mg 2+,
whereas the concentrations of C1- and total dissolved solids are presented in Figs. 10-19
and 20, respectively. These results indicate that the total concentration of expelled
solutions goes through a maximum before starting to decrease with increasing pressure.
The concentrations of Ca 2+ and Mg 2+ ions in squeezed-out solutions, however, start to
increase again during the latest stages of compaction. This can possibly be attributed to