Page 285 - Origin and Prediction of Abnormal Formation Pressures
P. 285
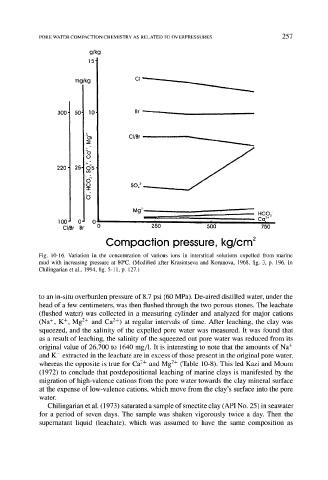
PORE WATER COMPACTION CHEMISTRY AS RELATED TO OVERPRESSURES 257
g/kg
mg/kg C l ~
300 50 10 Br
I00 0" 0 , Ca~ §
CI/Br Br- 0 250 5~30 750
Compaction pressure, kg/cm
Fig. 10-16. Variation in the concentration of various ions in interstitial solutions expelled from marine
mud with increasing pressure at 80~ (Modified after Krasintseva and Korunova, 1968, fig. 3, p. 196. In
Chilingarian et al., 1994, fig. 5-11, p. 127.)
to an in-situ overburden pressure of 8.7 psi (60 MPa). De-aired distilled water, under the
head of a few centimeters, was then flushed through the two porous stones. The leachate
(flushed water) was collected in a measuring cylinder and analyzed for major cations
(Na +, K +, Mg 2+ and Ca 2+) at regular intervals of time. After leaching, the clay was
squeezed, and the salinity of the expelled pore water was measured. It was found that
as a result of leaching, the salinity of the squeezed out pore water was reduced from its
original value of 26,700 to 1640 mg/1. It is interesting to note that the amounts of Na +
and K + extracted in the leachate are in excess of those present in the original pore water,
whereas the opposite is true for Ca 2+ and Mg 2+ (Table 10-8). This led Kazi and Moum
(1972) to conclude that postdepositional leaching of marine clays is manifested by the
migration of high-valence cations from the pore water towards the clay mineral surface
at the expense of low-valence cations, which move from the clay's surface into the pore
water.
Chilingarian et al. (1973) saturated a sample of smectite clay (API No. 25) in seawater
for a period of seven days. The sample was shaken vigorously twice a day. Then the
supernatant liquid (leachate), which was assumed to have the same composition as