Page 281 - Origin and Prediction of Abnormal Formation Pressures
P. 281
PORE WATER COMPACTION CHEMISTRY AS RELATED TO OVERPRESSURES 253
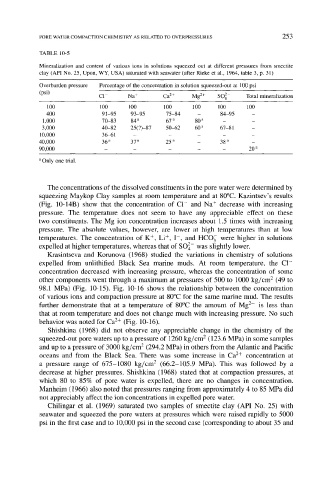
TABLE 10-5
Mineralization and content of various ions in solutions squeezed out at different pressures from smectite
clay (API No. 25, Upon, WY, USA) saturated with seawater (after Rieke et al., 1964, table 3, p. 31)
Overburden pressure Percentage of the concentration in solution squeezed-out at 100 psi
(psi) C1- Na + Ca 2+ Mg 2+ SO 2- Total mineralization
100 100 100 100 100 100 100
400 91-95 93-95 75-84 - 84-95 -
1,000 70-83 84 a 67 a 80 a _ _
3,000 40-82 25(?)-87 50-62 60 a 67-81 -
10,000 36-61 . . . . .
40,000 36 a 37 a 25 a - 38 a _
90,000 . . . . . 20 a
a Only one trial.
The concentrations of the dissolved constituents in the pore water were determined by
squeezing Maykop Clay samples at room temperature and at 80~ Kazintsev's results
(Fig. 10-14B) show that the concentration of C1- and Na + decrease with increasing
pressure. The temperature does not seem to have any appreciable effect on these
two constituents. The Mg ion concentration increases about 1.5 times with increasing
pressure. The absolute values, however, are lower at high temperatures than at low
temperatures. The concentration of K +, Li +, I-, and HCO~- were higher in solutions
expelled at higher temperatures, whereas that of SO 2- was slightly lower.
Krasintseva and Korunova (1968) studied the variations in chemistry of solutions
expelled from unlithified Black Sea marine muds. At room temperature, the C1-
concentration decreased with increasing pressure, whereas the concentration of some
other components went through a maximum at pressures of 500 to 1000 kg/cm 2 (49 to
98.1 MPa) (Fig. 10-15). Fig. 10-16 shows the relationship between the concentration
of various ions and compaction pressure at 80~ for the same marine mud. The results
further demonstrate that at a temperature of 80~ the amount of Mg 2+ is less than
that at room temperature and does not change much with increasing pressure. No such
behavior was noted for Ca 2+ (Fig. 10-16).
Shishkina (1968) did not observe any appreciable change in the chemistry of the
squeezed-out pore waters up to a pressure of 1260 kg/cm 2 (123.6 MPa) in some samples
and up to a pressure of 3000 kg/cm 2 (294.2 MPa) in others from the Atlantic and Pacific
oceans and from the Black Sea. There was some increase in Ca 2+ concentration at
a pressure range of 675-1080 kg/cm 2 (66.2-105.9 MPa). This was followed by a
decrease at higher pressures. Shishkina (1968) stated that at compaction pressures, at
which 80 to 85% of pore water is expelled, there are no changes in concentration.
Manheim (1966) also noted that pressures ranging from approximately 4 to 85 MPa did
not appreciably affect the ion concentrations in expelled pore water.
Chilingar et al. (1969) saturated two samples of smectite clay (API No. 25) with
seawater and squeezed the pore waters at pressures which were raised rapidly to 5000
psi in the first case and to 10,000 psi in the second case (corresponding to about 35 and