Page 282 - Origin and Prediction of Abnormal Formation Pressures
P. 282
254 H.H. RIEKE, G.V. CHILINGAR AND J.O. ROBERTSON JR.
100 If~ No +
,,,..
D. Ca ++
0 80 9 Mg ++
0
Q
60
,.li..
0
"13 40
U
20
c- 0 b
O 100 l,O00 l 0,000 100,000
Overburden pressure, psi
.,,i,.
0
100 I~ cr
I So4 2-
c-
O 80
ii.. 1
p
c" 60
0 9
c-
O 40
0
0 20
0 I
I,i,
OI O0 1,000 10,000 1 O0 000
a_
Overburden pressure, psi
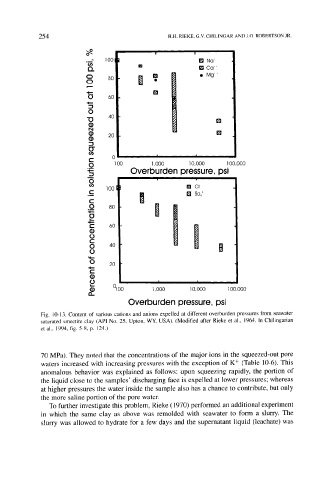
Fig. 10-13. Content of various cations and anions expelled at different overburden pressures from seawater
saturated smectite clay (API No. 25, Upton, WY, USA). (Modified after Rieke et al., 1964. In Chilingarian
et al., 1994, fig. 5-8, p. 124.)
70 MPa). They noted that the concentrations of the major ions in the squeezed-out pore
waters increased with increasing pressures with the exception of K + (Table 10-6). This
anomalous behavior was explained as follows: upon squeezing rapidly, the portion of
the liquid close to the samples' discharging face is expelled at lower pressures; whereas
at higher pressures the water inside the sample also has a chance to contribute, but only
the more saline portion of the pore water.
To further investigate this problem, Rieke (1970) performed an additional experiment
in which the same clay as above was remolded with seawater to form a slurry. The
slurry was allowed to hydrate for a few days and the supernatant liquid (leachate) was