Page 283 - Origin and Prediction of Abnormal Formation Pressures
P. 283
PORE WATER COMPACTION CHEMISTRY AS RELATED TO OVERPRESSURES 255
A
I II Ill IV V VI VII
600 , , ,
s00
. i
O"
b~ 2 _
,._.,._. ~
1
0 4 6 8 10 12 14 16 18 20 22
Amount of solution squeezed out, g
B
mg/kg
mg-equiv./kg mg-equiv./kg
34 7O .' 85C I Mg ~*
~ s o : : r ~ '" 180, r .----''HB~ o.18. ,18 '1 18o 900
26 30, 650 ij
/ Hco,-rr} 14 140, ,/,,,f' '. ......... ,14o .... ~ Mg2§ )
/
/
18 90, 45s j/
o.,1o,, 1o i lOO 500.
1oo.
I r
~ 6 60, 6 _ _ - Sr
lO 50' 250 Jf
Sr 01
2 ;o 9 56 HCO; % c& +
2 20; 2 0.02 2 20. ,100,
3 6 9 112 15 18
HCO so~ ~- I i II iii II IV / v Vl S12 K ~ C F + N r ! ,,! "~,v ~~ :, '~
Mg 2§
g g g
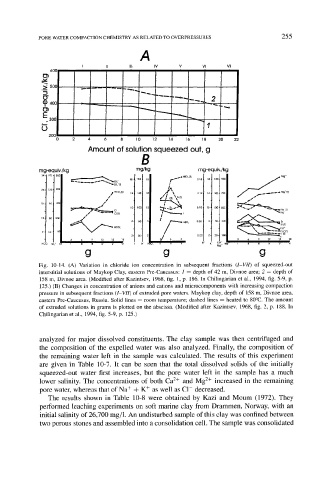
Fig. 10-14. (A) Variation in chloride ion concentration in subsequent fractions (I-VII) of squeezed-out
interstitial solutions of Maykop Clay, eastern Pre-Caucasus: 1 = depth of 42 m, Divnoe area; 2 -- depth of
158 m, Divnoe area. (Modified after Kazintsev, 1968, fig. 1, p. 186. In Chilingarian et al., 1994, fig. 5-9, p.
125.) (B) Changes in concentration of anions and cations and microcomponents with increasing compaction
pressure in subsequent fractions (I-VII) of extruded pore waters. Maykop clay, depth of 158 m, Divnoe area,
eastern Pre-Caucasus, Russia. Solid lines - room temperature; dashed lines = heated to 80~ The amount
of extruded solutions in grams is plotted on the abscissa. (Modified after Kazintsev, 1968, fig. 2, p. 188. In
Chilingarian et al., 1994, fig. 5-9, p. 125.)
analyzed for major dissolved constituents. The clay sample was then centrifuged and
the composition of the expelled water was also analyzed. Finally, the composition of
the remaining water left in the sample was calculated. The results of this experiment
are given in Table 10-7. It can be seen that the total dissolved solids of the initially
squeezed-out water first increases, but the pore water left in the sample has a much
lower salinity. The concentrations of both Ca 2+ and Mg 2+ increased in the remaining
pore water, whereas that of Na + 4- K + as well as C1- decreased.
The results shown in Table 10-8 were obtained by Kazi and Moum (1972). They
performed leaching experiments on soft marine clay from Drammen, Norway, with an
initial salinity of 26,700 mg/1. An undisturbed sample of this clay was confined between
two porous stones and assembled into a consolidation cell. The sample was consolidated