Page 280 - Origin and Prediction of Abnormal Formation Pressures
P. 280
252 H.H. RIEKE, G.V. CHILINGAR AND J.O. ROBERTSON JR.
+ A
200
n
E ]50
=4.-=.
(1) 2 I B
0 100 2
>
= ,,,.m 3
100
O"
E 5o 1~" 4o
i~ 20 4
. . . . 4 ._,,,._~. L
0 . . . . ~ ~ 5
0 . . . . . . . . ' ..... E 0 -,-.-,--
70 60 50 40 30 20 10 0 200 150 1 O0 50
" % H20 %/-/20
-
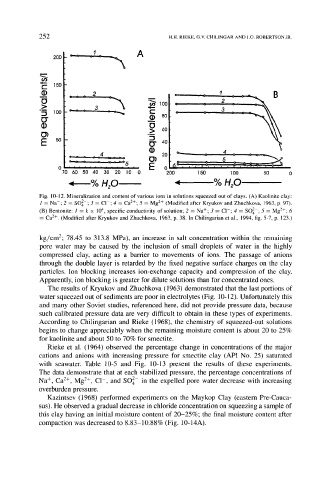
Fig. 10-12. Mineralization and content of various ions in solutions squeezed out of clays. (A) Kaolinite clay:
1 -- Na +" 2 = SO]-" 3 = CI-' 4 -- Ca 2+" 5 = Mg 2+ (Modified after Kryukov and Zhuchkova, 1963, p. 97).
(B) Bentonite: 1 = k x 104, specific conductivity of solution; 2 - Na +" 3 = C1-" 4 -- SO]-; 5 = Mg 2+" 6
-- Ca 2+. (Modified after Kryukov and Zhuchkova, 1963, p. 38. In Chilingarian et al., 1994, fig. 5-7, p. 123.)
kg/cm2; 78.45 to 313.8 MPa), an increase in salt concentration within the remaining
pore water may be caused by the inclusion of small droplets of water in the highly
compressed clay, acting as a barrier to movements of ions. The passage of anions
through the double layer is retarded by the fixed negative surface charges on the clay
particles. Ion blocking increases ion-exchange capacity and compression of the clay.
Apparently, ion blocking is greater for dilute solutions than for concentrated ones.
The results of Kryukov and Zhuchkova (1963) demonstrated that the last portions of
water squeezed out of sediments are poor in electrolytes (Fig. 10-12). Unfortunately this
and many other Soviet studies, referenced here, did not provide pressure data, because
such calibrated pressure data are very difficult to obtain in these types of experiments.
According to Chilingarian and Rieke (1968), the chemistry of squeezed-out solutions
begins to change appreciably when the remaining moisture content is about 20 to 25%
for kaolinite and about 50 to 70% for smectite.
Rieke et al. (1964) observed the percentage change in concentrations of the major
cations and anions with increasing pressure for smectite clay (API No. 25) saturated
with seawater. Table 10-5 and Fig. 10-13 present the results of these experiments.
The data demonstrate that at each stabilized pressure, the percentage concentrations of
Na +, Ca 2+, Mg 2+, CI-, and SO 2- in the expelled pore water decrease with increasing
overburden pressure.
Kazintsev (1968) performed experiments on the Maykop Clay (eastern Pre-Cauca-
sus). He observed a gradual decrease in chloride concentration on squeezing a sample of
this clay having an initial moisture content of 20-25%; the final moisture content after
compaction was decreased to 8.83-10.88% (Fig. 10-14A).