Page 141 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 141
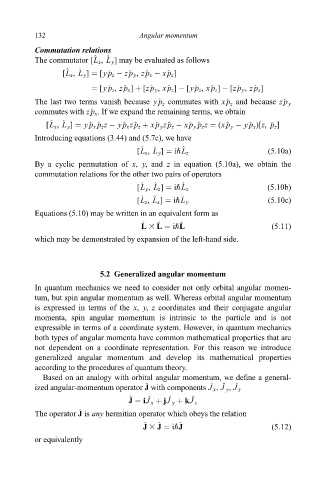
132 Angular momentum
Commutation relations
^
^
The commutator [L x , L y ] may be evaluated as follows
^
^
[L x , L y ] [y^ p z ÿ z^ p y , z^ p x ÿ x^ p z ]
[y^ p z , z^ p x ] [z^ p y , x^ p z ] ÿ [y^ p z , x^ p z ] ÿ [z^ p y , z^ p x ]
The last two terms vanish because y^ p z commutes with x^ p z and because z^ p y
commutes with z^ p x . If we expand the remaining terms, we obtain
^
^
[L x , L y ] y^ p x ^ p z z ÿ y^ p x z^ p z x^ p y z^ p z ÿ x^ p y ^ p z z (x^ p y ÿ y^ p x )[z, ^ p z ]
Introducing equations (3.44) and (5.7c), we have
^ ^ ^
[L x , L y ] i"L z (5:10a)
By a cyclic permutation of x, y, and z in equation (5.10a), we obtain the
commutation relations for the other two pairs of operators
^ ^ ^
[L y , L z ] i"L x (5:10b)
^ ^ ^
[L z , L x ] i"L y (5:10c)
Equations (5.10) may be written in an equivalent form as
^ ^ ^ (5:11)
L 3 L i"L
which may be demonstrated by expansion of the left-hand side.
5.2 Generalized angular momentum
In quantum mechanics we need to consider not only orbital angular momen-
tum, but spin angular momentum as well. Whereas orbital angular momentum
is expressed in terms of the x, y, z coordinates and their conjugate angular
momenta, spin angular momentum is intrinsic to the particle and is not
expressible in terms of a coordinate system. However, in quantum mechanics
both types of angular momenta have common mathematical properties that are
not dependent on a coordinate representation. For this reason we introduce
generalized angular momentum and develop its mathematical properties
according to the procedures of quantum theory.
Based on an analogy with orbital angular momentum, we de®ne a general-
^ ^ ^ ^
ized angular-momentum operator J with components J x , J y , J z
^ ^ ^ ^
J iJ x jJ y kJ z
^
The operator J is any hermitian operator which obeys the relation
^ ^ ^ (5:12)
J 3 J i"J
or equivalently