Page 166 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 166
6.1 Two-particle problem 157
hí, Bohr was able to reproduce the
the Planck relation ÄE E n 1 ÿ E n 2
experimental spectral lines and obtain a theoretical value for the Rydberg
constant that agrees exactly with the experimentally determined value. Further
investigations, however, showed that the Bohr model is not an accurate
representation of the hydrogen-atom structure, even though it gives the correct
formula for the energy levels, and led eventually to Schrodinger's wave mech-
È
È
anics. Schrodinger also used the hydrogen atom to illustrate his new theory.
6.1 Two-particle problem
In order to apply quantum-mechanical theory to the hydrogen atom, we ®rst
È
need to ®nd the appropriate Hamiltonian operator and Schrodinger equation.
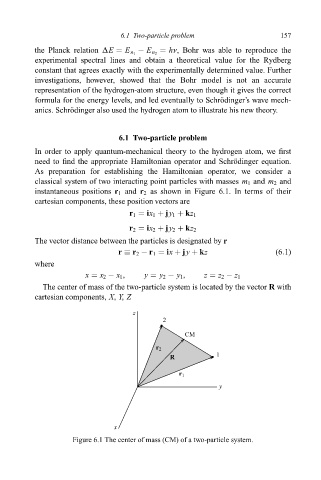
As preparation for establishing the Hamiltonian operator, we consider a
classical system of two interacting point particles with masses m 1 and m 2 and
instantaneous positions r 1 and r 2 as shown in Figure 6.1. In terms of their
cartesian components, these position vectors are
r 1 ix 1 jy 1 kz 1
r 2 ix 2 jy 2 kz 2
The vector distance between the particles is designated by r
r r 2 ÿ r 1 ix jy kz (6:1)
where
x x 2 ÿ x 1 , y y 2 ÿ y 1 , z z 2 ÿ z 1
The center of mass of the two-particle system is located by the vector R with
cartesian components, X, Y, Z
z
2
CM
r 2
1
R
r 1
y
x
Figure 6.1 The center of mass (CM) of a two-particle system.