Page 167 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 167
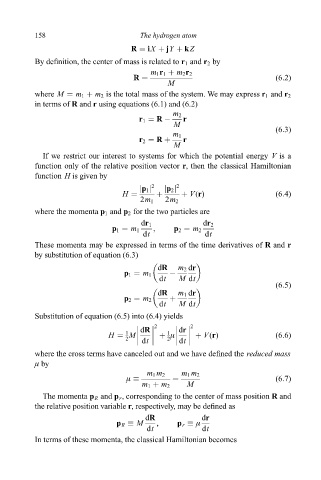
158 The hydrogen atom
R iX jY kZ
By de®nition, the center of mass is related to r 1 and r 2 by
m 1 r 1 m 2 r 2
R (6:2)
M
where M m 1 m 2 is the total mass of the system. We may express r 1 and r 2
in terms of R and r using equations (6.1) and (6.2)
m 2
r 1 R ÿ r
M
(6:3)
m 1
r 2 R r
M
If we restrict our interest to systems for which the potential energy V is a
function only of the relative position vector r, then the classical Hamiltonian
function H is given by
2 2
jp 1 j jp 2 j
H V(r) (6:4)
2m 1 2m 2
where the momenta p 1 and p 2 for the two particles are
dr 1 dr 2
p 1 m 1 , p 2 m 2
dt dt
These momenta may be expressed in terms of the time derivatives of R and r
by substitution of equation (6.3)
dR m 2 dr
p 1 m 1 ÿ
dt M dt
(6:5)
dR m 1 dr
p 2 m 2
dt M dt
Substitution of equation (6.5) into (6.4) yields
2 2
dR dr
1
H M ì V(r) (6:6)
1
2 dt 2 dt
where the cross terms have canceled out and we have de®ned the reduced mass
ì by
m 1 m 2 m 1 m 2
ì (6:7)
m 1 m 2 M
The momenta p R and p r , corresponding to the center of mass position R and
the relative position variable r, respectively, may be de®ned as
dR dr
p R M , p r ì
dt dt
In terms of these momenta, the classical Hamiltonian becomes