Page 163 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 163
154 Angular momentum
B
dL
dt
Lsinθ
L
θ
v
r
I
M
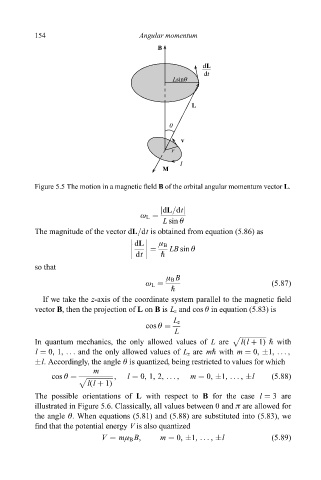
Figure 5.5 The motion in a magnetic ®eld B of the orbital angular momentum vector L.
jdL=dtj
ù L
L sin è
The magnitude of the vector dL=dt is obtained from equation (5.86) as
dL ì B
LB sin è
dt
"
so that
ì B B
ù L (5:87)
"
If we take the z-axis of the coordinate system parallel to the magnetic ®eld
vector B, then the projection of L on B is L z and cos è in equation (5.83) is
L z
cos è
L
p
In quantum mechanics, the only allowed values of L are l(l 1) " with
l 0, 1, ... and the only allowed values of L z are m" with m 0, 1, ... ,
l. Accordingly, the angle è is quantized, being restricted to values for which
m
, l 0, 1, 2, ... , m 0, 1, ... , l (5:88)
cos è p
l(l 1)
The possible orientations of L with respect to B for the case l 3 are
illustrated in Figure 5.6. Classically, all values between 0 and ð are allowed for
the angle è. When equations (5.81) and (5.88) are substituted into (5.83), we
®nd that the potential energy V is also quantized
V mì B B, m 0, 1, ... , l (5:89)