Page 159 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 159
150 Angular momentum
^
Accordingly, the quantum-mechanical Hamiltonian operator H for this system
^ 2
is proportional to the square of the angular momentum operator L
1
^ ^ 2
H L (5:72)
2I
^
^ 2
Thus, the operators H and L have the same eigenfunctions, namely, the
spherical harmonics Y Jm (è, j) as given in equation (5.50). It is customary in
discussions of the rigid rotor to replace the quantum number l by the index J in
the eigenfunctions and eigenvalues.
^
The eigenvalues of H are obtained by noting that
^ 1 ^ 2 J(J 1)" 2
HY Jm (è, j) L Y Jm (è, j) Y Jm (è, j) (5:73)
2I 2I
where l is replaced by J in equation (5.28a). Thus, the energy levels E J for the
rigid rotor are given by
" 2
E J J(J 1) J(J 1)B, J 0, 1, 2, ... (5:74)
2I
2
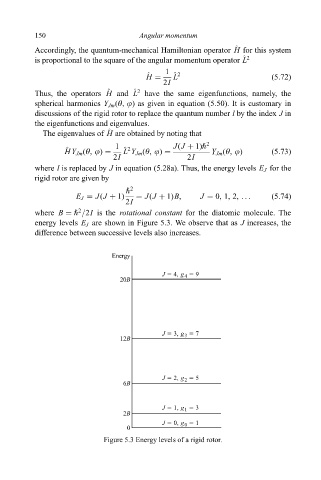
where B " =2I is the rotational constant for the diatomic molecule. The
energy levels E J are shown in Figure 5.3. We observe that as J increases, the
difference between successive levels also increases.
Energy
J 5 4, g 5 9
4
20B
J 5 3, g 5 7
3
12B
J 5 2, g 2 5 5
6B
5 3
J 5 1, g 1
2B
J 5 0, g 5 1
0
0
Figure 5.3 Energy levels of a rigid rotor.