Page 161 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 161
152 Angular momentum
ing current I enclosing a small area A gives rise to a magnetic moment M of
magnitude M given by
M IA (5:79)
2
The area A enclosed by the circular electronic orbit of radius r is ðr . From
2
equation (5.63) we have the relation L m e ùr . Thus, the magnitude of the
magnetic moment is related to the magnitude L of the angular momentum by
eL
M (5:80)
2m e
The direction of the vector L is determined by equation (5.62). By convention,
the direction of the current I is opposite to the direction of rotation of the
negatively charged electron, i.e., opposite to the direction of the vector v.
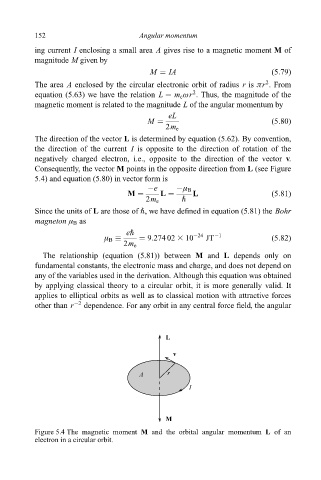
Consequently, the vector M points in the opposite direction from L (see Figure
5.4) and equation (5.80) in vector form is
ÿe ÿì B
M L L (5:81)
2m e "
Since the units of L are those of ", we have de®ned in equation (5.81) the Bohr
magneton ì B as
e" ÿ24 ÿ1
ì B 9:274 02 3 10 JT (5:82)
2m e
The relationship (equation (5.81)) between M and L depends only on
fundamental constants, the electronic mass and charge, and does not depend on
any of the variables used in the derivation. Although this equation was obtained
by applying classical theory to a circular orbit, it is more generally valid. It
applies to elliptical orbits as well as to classical motion with attractive forces
other than r ÿ2 dependence. For any orbit in any central force ®eld, the angular
L
v
A r
I
M
Figure 5.4 The magnetic moment M and the orbital angular momentum L of an
electron in a circular orbit.