Page 59 - PVT Property Correlations
P. 59
Dry Gases Chapter | 3 39
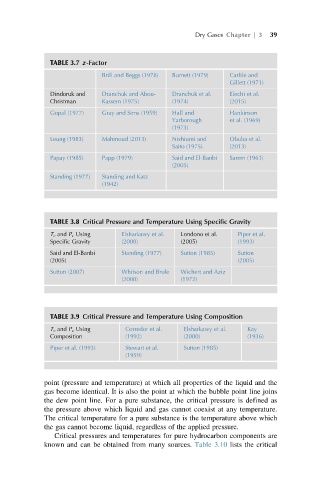
TABLE 3.7 z-Factor
Brill and Beggs (1978) Burnett (1979) Carlile and
Gillett (1971)
Dindoruk and Dranchuk and Abou- Dranchuk et al. Elechi et al.
Christman Kassem (1975) (1974) (2015)
Gopal (1977) Gray and Sims (1959) Hall and Hankinson
Yarborough et al. (1969)
(1973)
Leung (1983) Mahmoud (2013) Nishiumi and Obuba et al.
Saito (1975) (2013)
Papay (1985) Papp (1979) Said and El-Banbi Sarem (1961)
(2005)
Standing (1977) Standing and Katz
(1942)
TABLE 3.8 Critical Pressure and Temperature Using Specific Gravity
T c and P c Using Elsharkawy et al. Londono et al. Piper et al.
Specific Gravity (2000) (2005) (1993)
Said and El-Banbi Standing (1977) Sutton (1985) Sutton
(2005) (2005)
Sutton (2007) Whitson and Brule Wichert and Aziz
(2000) (1972)
TABLE 3.9 Critical Pressure and Temperature Using Composition
T c and P c Using Corredor et al. Elsharkawy et al. Kay
Composition (1992) (2000) (1936)
Piper et al. (1993) Stewart et al. Sutton (1985)
(1959)
point (pressure and temperature) at which all properties of the liquid and the
gas become identical. It is also the point at which the bubble point line joins
the dew point line. For a pure substance, the critical pressure is defined as
the pressure above which liquid and gas cannot coexist at any temperature.
The critical temperature for a pure substance is the temperature above which
the gas cannot become liquid, regardless of the applied pressure.
Critical pressures and temperatures for pure hydrocarbon components are
known and can be obtained from many sources. Table 3.10 lists the critical