Page 57 - PVT Property Correlations
P. 57
Dry Gases Chapter | 3 37
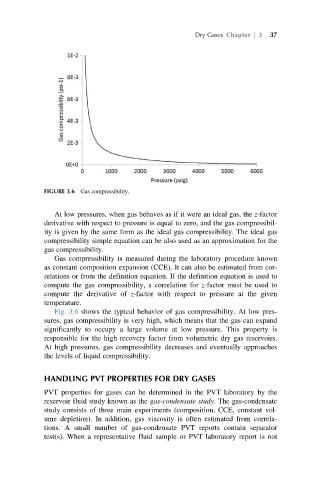
FIGURE 3.6 Gas compressibility.
At low pressures, when gas behaves as if it were an ideal gas, the z-factor
derivative with respect to pressure is equal to zero, and the gas compressibil-
ity is given by the same form as the ideal gas compressibility. The ideal gas
compressibility simple equation can be also used as an approximation for the
gas compressibility.
Gas compressibility is measured during the laboratory procedure known
as constant composition expansion (CCE). It can also be estimated from cor-
relations or from the definition equation. If the definition equation is used to
compute the gas compressibility, a correlation for z-factor must be used to
compute the derivative of z-factor with respect to pressure at the given
temperature.
Fig. 3.6 shows the typical behavior of gas compressibility. At low pres-
sures, gas compressibility is very high, which means that the gas can expand
significantly to occupy a large volume at low pressure. This property is
responsible for the high recovery factor from volumetric dry gas reservoirs.
At high pressures, gas compressibility decreases and eventually approaches
the levels of liquid compressibility.
HANDLING PVT PROPERTIES FOR DRY GASES
PVT properties for gases can be determined in the PVT laboratory by the
reservoir fluid study known as the gas-condensate study. The gas-condensate
study consists of three main experiments (composition, CCE, constant vol-
ume depletion). In addition, gas viscosity is often estimated from correla-
tions. A small number of gas-condensate PVT reports contain separator
test(s). When a representative fluid sample or PVT laboratory report is not