Page 52 - PVT Property Correlations
P. 52
32 PVT Property Correlations
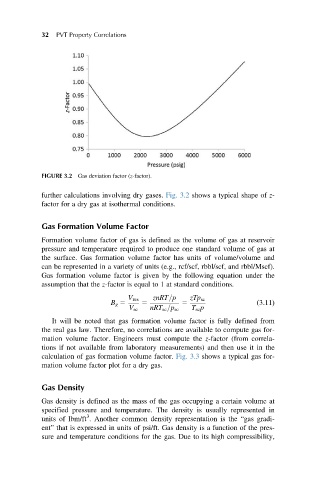
FIGURE 3.2 Gas deviation factor (z-factor).
further calculations involving dry gases. Fig. 3.2 shows a typical shape of z-
factor for a dry gas at isothermal conditions.
Gas Formation Volume Factor
Formation volume factor of gas is defined as the volume of gas at reservoir
pressure and temperature required to produce one standard volume of gas at
the surface. Gas formation volume factor has units of volume/volume and
can be represented in a variety of units (e.g., rcf/scf, rbbl/scf, and rbbl/Mscf).
Gas formation volume factor is given by the following equation under the
assumption that the z-factor is equal to 1 at standard conditions.
znRT=p
V res zTp sc
B g 5 5 5 ð3:11Þ
V sc nRT sc =p sc T sc p
It will be noted that gas formation volume factor is fully defined from
the real gas law. Therefore, no correlations are available to compute gas for-
mation volume factor. Engineers must compute the z-factor (from correla-
tions if not available from laboratory measurements) and then use it in the
calculation of gas formation volume factor. Fig. 3.3 shows a typical gas for-
mation volume factor plot for a dry gas.
Gas Density
Gas density is defined as the mass of the gas occupying a certain volume at
specified pressure and temperature. The density is usually represented in
3
units of lbm/ft . Another common density representation is the “gas gradi-
ent” that is expressed in units of psi/ft. Gas density is a function of the pres-
sure and temperature conditions for the gas. Due to its high compressibility,