Page 53 - PVT Property Correlations
P. 53
Dry Gases Chapter | 3 33
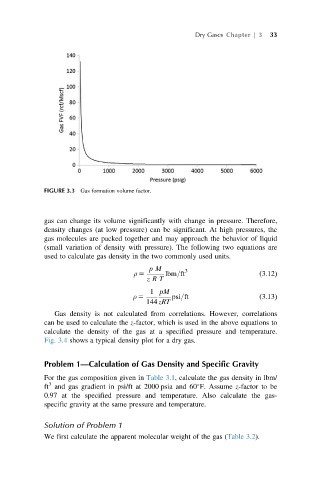
FIGURE 3.3 Gas formation volume factor.
gas can change its volume significantly with change in pressure. Therefore,
density changes (at low pressure) can be significant. At high pressures, the
gas molecules are packed together and may approach the behavior of liquid
(small variation of density with pressure). The following two equations are
used to calculate gas density in the two commonly used units.
pM 3
ρ 5 lbm=ft ð3:12Þ
zR T
1 pM
ρ 5 psi=ft ð3:13Þ
144 zRT
Gas density is not calculated from correlations. However, correlations
can be used to calculate the z-factor, which is used in the above equations to
calculate the density of the gas at a specified pressure and temperature.
Fig. 3.4 shows a typical density plot for a dry gas.
Problem 1—Calculation of Gas Density and Specific Gravity
For the gas composition given in Table 3.1, calculate the gas density in lbm/
3
ft and gas gradient in psi/ft at 2000 psia and 60 F. Assume z-factor to be
0.97 at the specified pressure and temperature. Also calculate the gas-
specific gravity at the same pressure and temperature.
Solution of Problem 1
We first calculate the apparent molecular weight of the gas (Table 3.2).