Page 130 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 130
SORPTION FROM WATER SOLUTION 121
capacity of DDT with SOM is far less than that of benzene. Further, the obser-
vation that polar organic liquids exhibit much higher uptake than nonpolar
organic liquids on a high-organic-content peat soil (Chiou and Kile, 1994) is
intrinsically consistent with solute partition rather than with hydrophobic
adsorption.
The sorption characteristics of individual solutes from a binary or multi-
solute system provide another means for distinction between partition and
adsorption. In their study of the simultaneous sorption of pyrene and phenan-
threne from water by river sediments, Karickhoff et al. (1979) observed no dis-
cernible sorptive interference between the two compounds (although no
explicit single-solute versus binary-solute data were presented). Similarly,
Chiou et al. (1983, 1985) found no apparent sorptive competition between
m-dichlorobenzene and 1,2,4-trichlorobenzene and between parathion and
lindane as binary solutes from water on soils over the concentrations investi-
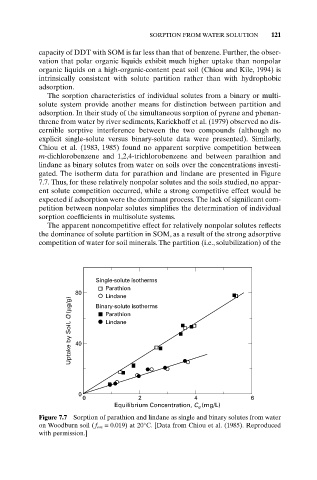
gated. The isotherm data for parathion and lindane are presented in Figure
7.7. Thus, for these relatively nonpolar solutes and the soils studied, no appar-
ent solute competition occurred, while a strong competitive effect would be
expected if adsorption were the dominant process. The lack of significant com-
petition between nonpolar solutes simplifies the determination of individual
sorption coefficients in multisolute systems.
The apparent noncompetitive effect for relatively nonpolar solutes reflects
the dominance of solute partition in SOM, as a result of the strong adsorptive
competition of water for soil minerals. The partition (i.e., solubilization) of the
Single-solute isotherms
Parathion
80 Binary-solute isotherms
Lindane
Uptake by Soil, Q (µg/g) 40 Parathion
Lindane
0
0 2 4 6
Equilibrium Concentration, C (mg/L)
e
Figure 7.7 Sorption of parathion and lindane as single and binary solutes from water
on Woodburn soil (f om = 0.019) at 20°C. [Data from Chiou et al. (1985). Reproduced
with permission.]