Page 126 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 126
SORPTION FROM WATER SOLUTION 117
The calculated molar heats of sorption for most organic compounds and
pesticides on soil in water are generally less exothermic than -DH w and are
largely independent of sorption capacities (Mills and Biggar, 1969; Yaron and
Saltzman, 1972; Pierce et al., 1974; Chiou et al., 1979, 1985). The same is true
of the sorption of organic compounds from the vapor phase by water-
saturated soils, such as found for ethylene dibromide (Wade, 1954) and lindane
(Spencer and Cliath, 1970), in which the heats of sorption are less exothermic
than the heats of vapor condensation (-DH v). These results are inherently con-
sistent with the conceived partition uptake of nonionic organic compounds by
the soil organic matter of water-saturated soils.
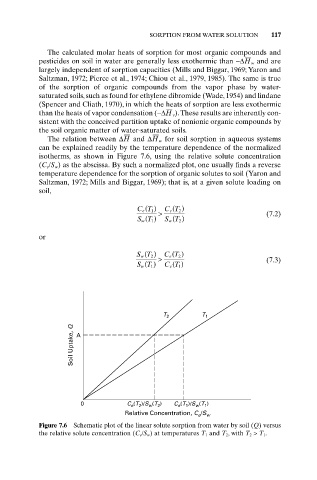
The relation between DH and DH w for soil sorption in aqueous systems
can be explained readily by the temperature dependence of the normalized
isotherms, as shown in Figure 7.6, using the relative solute concentration
(C e/S w) as the abscissa. By such a normalized plot, one usually finds a reverse
temperature dependence for the sorption of organic solutes to soil (Yaron and
Saltzman, 1972; Mills and Biggar, 1969); that is, at a given solute loading on
soil,
e () e ()
CT 1 > CT 2 (7.2)
w () w ()
ST 1 ST 2
or
w () e ()
ST 2 > CT 2 (7.3)
w () e ()
ST 1 CT 1
T
T 2 1
Soil Uptake, Q A
0 C (T )/S (T ) C (T )/S (T )
1
e
w
1
2
e
2
w
Relative Concentration, C /S w
e
Figure 7.6 Schematic plot of the linear solute sorption from water by soil (Q) versus
the relative solute concentration (C e /S w ) at temperatures T 1 and T 2 , with T 2 > T 1 .