Page 123 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 123
114 CONTAMINANT SORPTION TO SOILS AND NATURAL SOLIDS
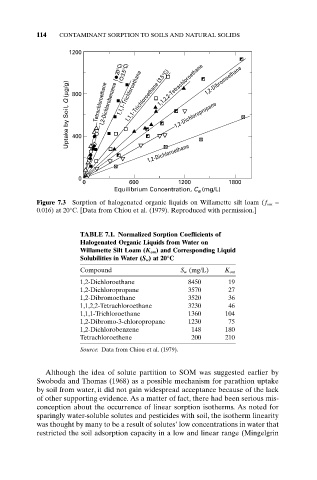
1200 1,2-Dibromoethane
1,1,2,2-Tetrachloroethane
1,1,1-Trichloroethane
Uptake by Soil, Q (µg/g) 800 Tetrachloroethene 1,2-Dichlorobenzene ( 20°C) ( 3.5°C) 1,1,1-Trichloroethane (3.5°C) 1,2-Dichloropropane
400
1,2-Dichloroethane
0
0 600 1200 1800
Equilibrium Concentration, C (mg/L)
e
Figure 7.3 Sorption of halogenated organic liquids on Willamette silt loam (f om =
0.016) at 20°C. [Data from Chiou et al. (1979). Reproduced with permission.]
TABLE 7.1. Normalized Sorption Coefficients of
Halogenated Organic Liquids from Water on
Willamette Silt Loam (K om) and Corresponding Liquid
Solubilities in Water (S w) at 20°C
Compound S w (mg/L) K om
1,2-Dichloroethane 8450 19
1,2-Dichloropropane 3570 27
1,2-Dibromoethane 3520 36
1,1,2,2-Tetrachloroethane 3230 46
1,1,1-Trichloroethane 1360 104
1,2-Dibromo-3-chloropropane 1230 75
1,2-Dichlorobenzene 148 180
Tetrachloroethene 200 210
Source: Data from Chiou et al. (1979).
Although the idea of solute partition to SOM was suggested earlier by
Swoboda and Thomas (1968) as a possible mechanism for parathion uptake
by soil from water, it did not gain widespread acceptance because of the lack
of other supporting evidence. As a matter of fact, there had been serious mis-
conception about the occurrence of linear sorption isotherms. As noted for
sparingly water-soluble solutes and pesticides with soil, the isotherm linearity
was thought by many to be a result of solutes’ low concentrations in water that
restricted the soil adsorption capacity in a low and linear range (Mingelgrin