Page 122 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 122
SORPTION FROM WATER SOLUTION 113
0 400 800 1200 1600
240 Benzene (upper and right scales)
1,3-Dichlorobenzene
Uptake by Soil, Q (µg/g) 160 600
1,2,4-Trichlorobenzene
80
400
200
0 0
0 20 40 60 80
Equilibrium Concentration, C (mg/L)
e
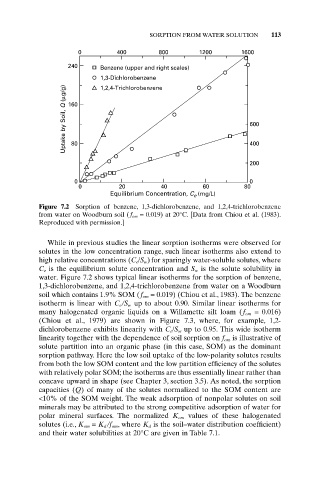
Figure 7.2 Sorption of benzene, 1,3-dichlorobenzene, and 1,2,4-trichlorobenzene
from water on Woodburn soil ( f om = 0.019) at 20°C. [Data from Chiou et al. (1983).
Reproduced with permission.]
While in previous studies the linear sorption isotherms were observed for
solutes in the low concentration range, such linear isotherms also extend to
high relative concentrations (C e /S w ) for sparingly water-soluble solutes, where
C e is the equilibrium solute concentration and S w is the solute solubility in
water. Figure 7.2 shows typical linear isotherms for the sorption of benzene,
1,3-dichlorobenzene, and 1,2,4-trichlorobenzene from water on a Woodburn
soil which contains 1.9% SOM (f om = 0.019) (Chiou et al., 1983). The benzene
isotherm is linear with C e /S w up to about 0.90. Similar linear isotherms for
many halogenated organic liquids on a Willamette silt loam (f om = 0.016)
(Chiou et al., 1979) are shown in Figure 7.3, where, for example, 1,2-
dichlorobenzene exhibits linearity with C e/S w up to 0.95. This wide isotherm
linearity together with the dependence of soil sorption on f om is illustrative of
solute partition into an organic phase (in this case, SOM) as the dominant
sorption pathway. Here the low soil uptake of the low-polarity solutes results
from both the low SOM content and the low partition efficiency of the solutes
with relatively polar SOM; the isotherms are thus essentially linear rather than
concave upward in shape (see Chapter 3, section 3.5). As noted, the sorption
capacities (Q) of many of the solutes normalized to the SOM content are
<10% of the SOM weight. The weak adsorption of nonpolar solutes on soil
minerals may be attributed to the strong competitive adsorption of water for
polar mineral surfaces. The normalized K om values of these halogenated
solutes (i.e., K om = K d /f om, where K d is the soil–water distribution coefficient)
and their water solubilities at 20°C are given in Table 7.1.