Page 124 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 124
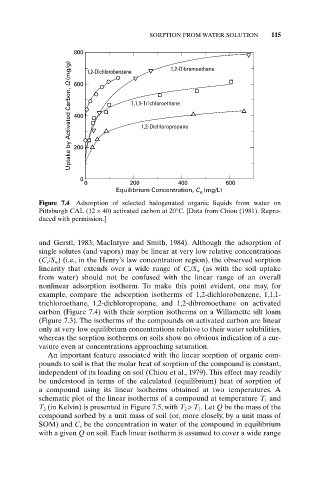
SORPTION FROM WATER SOLUTION 115
800 1,2-Dichlorobenzene 1,2-Dibromoethane
Uptake by Activated Carbon, Q (mg/g) 400 1,1,1-Trichloroethane
600
1,2-Dichloropropane
200
0
0 200 400 600
Equilibrium Concentration, C (mg/L)
e
Figure 7.4 Adsorption of selected halogenated organic liquids from water on
Pittsburgh CAL (12 ¥ 40) activated carbon at 20°C. [Data from Chiou (1981). Repro-
duced with permission.]
and Gerstl, 1983; MacIntyre and Smith, 1984). Although the adsorption of
single solutes (and vapors) may be linear at very low relative concentrations
(C e /S w ) (i.e., in the Henry’s law concentration region), the observed sorption
linearity that extends over a wide range of C e /S w (as with the soil uptake
from water) should not be confused with the linear range of an overall
nonlinear adsorption isotherm. To make this point evident, one may, for
example, compare the adsorption isotherms of 1,2-dichlorobenzene, 1,1,1-
trichloroethane, 1,2-dichloropropane, and 1,2-dibromoethane on activated
carbon (Figure 7.4) with their sorption isotherms on a Willamette silt loam
(Figure 7.3). The isotherms of the compounds on activated carbon are linear
only at very low equilibrium concentrations relative to their water solubilities,
whereas the sorption isotherms on soils show no obvious indication of a cur-
vature even at concentrations approaching saturation.
An important feature associated with the linear sorption of organic com-
pounds to soil is that the molar heat of sorption of the compound is constant,
independent of its loading on soil (Chiou et al., 1979). This effect may readily
be understood in terms of the calculated (equilibrium) heat of sorption of
a compound using its linear isotherms obtained at two temperatures. A
schematic plot of the linear isotherms of a compound at temperature T 1 and
T 2 (in Kelvin) is presented in Figure 7.5, with T 2 > T 1 . Let Q be the mass of the
compound sorbed by a unit mass of soil (or, more closely, by a unit mass of
SOM) and C e be the concentration in water of the compound in equilibrium
with a given Q on soil. Each linear isotherm is assumed to cover a wide range