Page 149 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 149
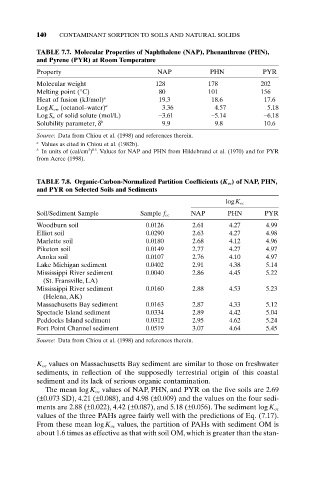
140 CONTAMINANT SORPTION TO SOILS AND NATURAL SOLIDS
TABLE 7.7. Molecular Properties of Naphthalene (NAP), Phenanthrene (PHN),
and Pyrene (PYR) at Room Temperature
Property NAP PHN PYR
Molecular weight 128 178 202
Melting point (°C) 80 101 156
Heat of fusion (kJ/mol) a 19.3 18.6 17.6
LogK ow (octanol–water) a 3.36 4.57 5.18
LogS w of solid solute (mol/L) -3.61 -5.14 -6.18
Solubility parameter, d b 9.9 9.8 10.6
Source: Data from Chiou et al. (1998) and references therein.
a Values as cited in Chiou et al. (1982b).
3 0.5
b In units of (cal/cm ) . Values for NAP and PHN from Hildebrand et al. (1970) and for PYR
from Acree (1998).
TABLE 7.8. Organic-Carbon-Normalized Partition Coefficients (K oc) of NAP, PHN,
and PYR on Selected Soils and Sediments
logK oc
Soil/Sediment Sample Sample f oc NAP PHN PYR
Woodburn soil 0.0126 2.61 4.27 4.99
Elliot soil 0.0290 2.63 4.27 4.98
Marlette soil 0.0180 2.68 4.12 4.96
Piketon soil 0.0149 2.77 4.27 4.97
Anoka soil 0.0107 2.76 4.10 4.97
Lake Michigan sediment 0.0402 2.91 4.38 5.14
Mississippi River sediment 0.0040 2.86 4.45 5.22
(St. Fransville, LA)
Mississippi River sediment 0.0160 2.88 4.53 5.23
(Helena, AK)
Massachusetts Bay sediment 0.0163 2.87 4.33 5.12
Spectacle Island sediment 0.0334 2.89 4.42 5.04
Peddocks Island sediment 0.0312 2.95 4.62 5.24
Fort Point Channel sediment 0.0519 3.07 4.64 5.45
Source: Data from Chiou et al. (1998) and references therein.
K oc values on Massachusetts Bay sediment are similar to those on freshwater
sediments, in reflection of the supposedly terrestrial origin of this coastal
sediment and its lack of serious organic contamination.
The mean logK oc values of NAP, PHN, and PYR on the five soils are 2.69
(±0.073 SD), 4.21 (±0.088), and 4.98 (±0.009) and the values on the four sedi-
ments are 2.88 (±0.022), 4.42 (±0.087), and 5.18 (±0.056). The sediment logK oc
values of the three PAHs agree fairly well with the predictions of Eq. (7.17).
From these mean logK oc values, the partition of PAHs with sediment OM is
about 1.6 times as effective as that with soil OM, which is greater than the stan-