Page 147 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 147
138 CONTAMINANT SORPTION TO SOILS AND NATURAL SOLIDS
correlation, which is relatively insensitive to solute polarity for the reasons
stated in Chapter 5, the logK om versus logK ow (or logK om versus logS w)
correlation is much more sensitive to solute polarity. In this sense, weakly
polar octanol (or K ow) is not a good replicate model for relatively polar SOM
(or K om).
7.3.4 Behavior of PAHs versus Other Nonpolar Contaminants
Although the K oc values of many nonpolar contaminants can be estimated with
sufficient accuracy from their physicochemical properties, such as K ow or S w,
the selection of a proper correlation for estimation is, however, complicated
by the inconsistency of published correlations for selected nonpolar com-
pounds on soils and sediments. A particular case in point is the inconsistency
of the correlations for polycyclic aromatic hydrocarbons (PAHs) relative to
other nonpolar organic compounds. For instance, with K oc = 1.85K om, the log
K oc versus logK ow correlation established by Chiou et al. (1983) [i.e., Eq.
(7.14)] for substituted aromatic compounds (primarily, chlorinated benzenes
and PCBs) on a soil over the range logK ow = 2.11 to 5.62 gives
logK oc = 0.904logK ow - 0.512 (7.16)
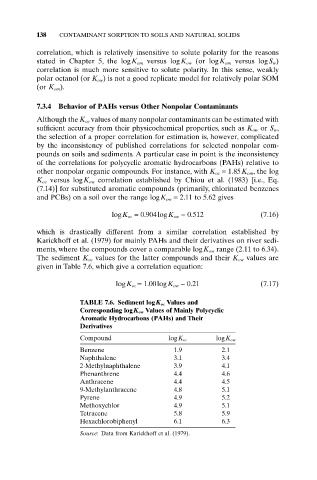
which is drastically different from a similar correlation established by
Karickhoff et al. (1979) for mainly PAHs and their derivatives on river sedi-
ments, where the compounds cover a comparable logK ow range (2.11 to 6.34).
The sediment K oc values for the latter compounds and their K ow values are
given in Table 7.6, which give a correlation equation:
logK oc = 1.00logK ow - 0.21 (7.17)
TABLE 7.6. Sediment logK oc Values and
Corresponding logK ow Values of Mainly Polycyclic
Aromatic Hydrocarbons (PAHs) and Their
Derivatives
Compound logK oc logK ow
Benzene 1.9 2.1
Naphthalene 3.1 3.4
2-Methylnaphthalene 3.9 4.1
Phenanthrene 4.4 4.6
Anthracene 4.4 4.5
9-Methylanthracene 4.8 5.1
Pyrene 4.9 5.2
Methoxychlor 4.9 5.1
Tetracene 5.8 5.9
Hexachlorobiphenyl 6.1 6.3
Source: Data from Karickhoff et al. (1979).