Page 146 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 146
SORPTION FROM WATER SOLUTION 137
4.5
Low-polarity compounds
High-polarity compounds
3.5
Log K om 2.5
1.5
0.5
0.5 2.5 4.5 6.5
Log K
ow
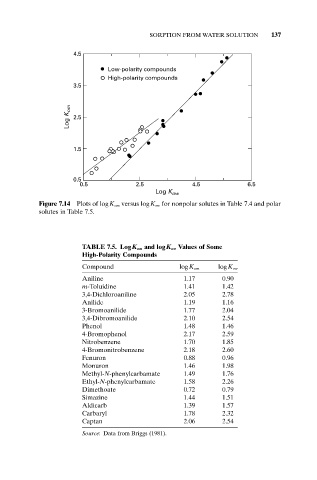
Figure 7.14 Plots of logK om versus logK ow for nonpolar solutes in Table 7.4 and polar
solutes in Table 7.5.
TABLE 7.5. LogK om and logK ow Values of Some
High-Polarity Compounds
Compound logK om logK ow
Aniline 1.17 0.90
m-Toluidine 1.41 1.42
3,4-Dichloroaniline 2.05 2.78
Anilide 1.19 1.16
3-Bromoanilide 1.77 2.04
3,4-Dibromoanilide 2.10 2.54
Phenol 1.48 1.46
4-Bromophenol 2.17 2.59
Nitrobenzene 1.70 1.85
4-Bromonitrobenzene 2.18 2.60
Fenuron 0.88 0.96
Monuron 1.46 1.98
Methyl-N-phenylcarbamate 1.49 1.76
Ethyl-N-phenylcarbamate 1.58 2.26
Dimethoate 0.72 0.79
Simazine 1.44 1.51
Aldicarb 1.39 1.57
Carbaryl 1.78 2.32
Captan 2.06 2.54
Source: Data from Briggs (1981).