Page 66 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 66
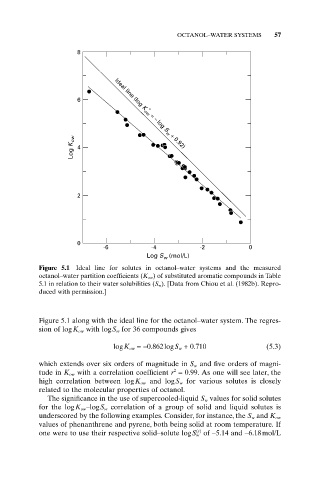
OCTANOL–WATER SYSTEMS 57
8
6
°
Log K ow 4
Ideal line (log K ow = – log S w + 0.92)
2
0
-6 -4 -2 0
Log S (mol/L)
w
Figure 5.1 Ideal line for solutes in octanol–water systems and the measured
octanol–water partition coefficients (K ow ) of substituted aromatic compounds in Table
5.1 in relation to their water solubilities (S w ). [Data from Chiou et al. (1982b). Repro-
duced with permission.]
Figure 5.1 along with the ideal line for the octanol–water system. The regres-
sion of logK ow with logS w for 36 compounds gives
logK ow =-0.862logS w + 0.710 (5.3)
which extends over six orders of magnitude in S w and five orders of magni-
2
tude in K ow with a correlation coefficient r = 0.99. As one will see later, the
high correlation between logK ow and logS w for various solutes is closely
related to the molecular properties of octanol.
The significance in the use of supercooled-liquid S w values for solid solutes
for the logK ow–logS w correlation of a group of solid and liquid solutes is
underscored by the following examples. Consider, for instance, the S w and K ow
values of phenanthrene and pyrene, both being solid at room temperature. If
(s)
one were to use their respective solid–solute logS w of -5.14 and -6.18mol/L