Page 70 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 70
HEPTANE–WATER SYSTEMS 61
8
6
°
4
Log K hw
Ideal line (log K hw = – log S w + 0.836)
2
0
-2
-6 -4 -2 0
Log S (mol/L)
w
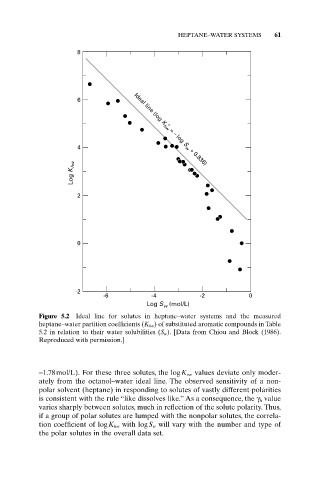
Figure 5.2 Ideal line for solutes in heptane–water systems and the measured
heptane–water partition coefficients (K hw ) of substituted aromatic compounds in Table
5.2 in relation to their water solubilities (S w ). [Data from Chiou and Block (1986).
Reproduced with permission.]
-1.78mol/L). For these three solutes, the logK ow values deviate only moder-
ately from the octanol–water ideal line. The observed sensitivity of a non-
polar solvent (heptane) in responding to solutes of vastly different polarities
is consistent with the rule “like dissolves like.” As a consequence, the g h value
varies sharply between solutes, much in reflection of the solute polarity. Thus,
if a group of polar solutes are lumped with the nonpolar solutes, the correla-
tion coefficient of logK hw with logS w will vary with the number and type of
the polar solutes in the overall data set.