Page 74 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 74
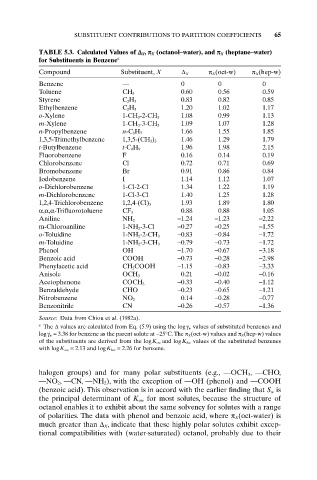
SUBSTITUENT CONTRIBUTIONS TO PARTITION COEFFICIENTS 65
TABLE 5.3. Calculated Values of D X , p X (octanol–water), and p X (heptane–water)
for Substituents in Benzene a
Compound Substituent, X D X p X (oct-w) p X (hep-w)
Benzene — 0 0 0
Toluene CH 3 0.60 0.56 0.59
Styrene C 2 H 3 0.83 0.82 0.85
Ethylbenzene C 2 H 5 1.20 1.02 1.17
o-Xylene 1-CH 3 -2-CH 3 1.08 0.99 1.13
m-Xylene 1-CH 3 -3-CH 3 1.09 1.07 1.28
n-Propylbenzene n-C 3 H 7 1.66 1.55 1.85
1,3,5-Trimethylbenzene 1,3,5-(CH 3 ) 3 1.46 1.29 1.79
t-Butylbenzene t-C 4 H 9 1.96 1.98 2.15
Fluorobenzene F 0.16 0.14 0.19
Chlorobenzene Cl 0.72 0.71 0.69
Bromobenzene Br 0.91 0.86 0.84
Iodobenzene I 1.14 1.12 1.07
o-Dichlorobenzene 1-Cl-2-Cl 1.34 1.22 1.19
m-Dichlorobenzene 1-Cl-3-Cl 1.40 1.25 1.28
1,2,4-Trichlorobenzene 1,2,4-(Cl) 3 1.93 1.89 1.80
a,a,a-Trifluorotoluene CF 3 0.88 0.88 1.05
Aniline NH 2 -1.24 -1.23 -2.22
m-Chloroaniline 1-NH 2-3-Cl -0.27 -0.25 -1.55
o-Toluidine 1-NH 2 -2-CH 3 -0.83 -0.84 -1.72
m-Toluidine 1-NH 2 -3-CH 3 -0.79 -0.73 -1.72
Phenol OH -1.70 -0.67 -3.18
Benzoic acid COOH -0.73 -0.28 -2.98
Phenylacetic acid CH 2 COOH -1.15 -0.83 -3.33
Anisole OCH 3 0.21 -0.02 -0.16
Acetophenone COCH 3 -0.33 -0.40 -1.12
Benzaldehyde CHO -0.23 -0.65 -1.21
Nitrobenzene NO 2 0.14 -0.28 -0.77
Benzonitrile CN -0.26 -0.57 -1.36
Source: Data from Chiou et al. (1982a).
a The D values are calculated from Eq. (5.9) using the logg w values of substituted benzenes and
logg w = 3.38 for benzene as the parent solute at ~25°C. The p X (oct-w) values and p X (hep-w) values
of the substituents are derived from the logK ow and logK hw values of the substituted benzenes
with logK ow = 2.13 and logK hw = 2.26 for benzene.
halogen groups) and for many polar substituents (e.g., —OCH 3 , —CHO,
—NO 2 , —CN, —NH 2 ), with the exception of —OH (phenol) and —COOH
(benzoic acid). This observation is in accord with the earlier finding that S w is
the principal determinant of K ow for most solutes, because the structure of
octanol enables it to exhibit about the same solvency for solutes with a range
of polarities. The data with phenol and benzoic acid, where p X (oct-water) is
much greater than D X , indicate that these highly polar solutes exhibit excep-
tional compatibilities with (water-saturated) octanol, probably due to their