Page 75 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 75
66 CONTAMINANT PARTITION AND BIOCONCENTRATION
t - C H
4 9
2
1,2,4 - Cl 3
1,3,5 - (CH ) n - C H
3 3
3 7
1,2 - Cl 2
1,3 - (CH ) l
3 2
1,3 - Cl 2
CF 3 C H
2 5
CH = CH 2 Cl Br 1,2 - (CH )
3 2
x (octanol-water) 0 1-NH -3-Cl F OCH 3 CH 3
2
π OH COOH COCH 3 CN NO 2
CHO
-3-CH
CH COOH 1-NH 2 3
2
1-NH -2-CH 3
2
NH 2
-2
-2 0 2
∆ x
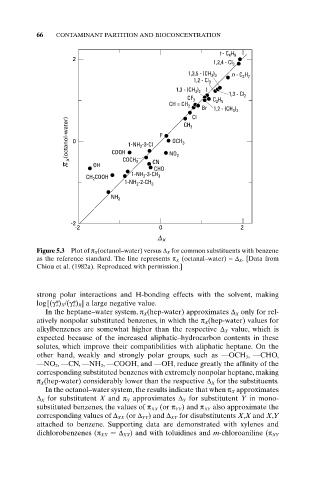
Figure 5.3 Plot of p X (octanol–water) versus D X for common substituents with benzene
as the reference standard. The line represents p X (octanal–water) =D X. [Data from
Chiou et al. (1982a). Reproduced with permission.]
strong polar interactions and H-bonding effects with the solvent, making
log[(g* o) X/(g* o) R] a large negative value.
In the heptane–water system, p X(hep-water) approximates D X only for rel-
atively nonpolar substituted benzenes, in which the p X(hep-water) values for
alkylbenzenes are somewhat higher than the respective D X value, which is
expected because of the increased aliphatic–hydrocarbon contents in these
solutes, which improve their compatibilities with aliphatic heptane. On the
other hand, weakly and strongly polar groups, such as —OCH 3 , —CHO,
—NO 2 , —CN, —NH 2 , —COOH, and —OH, reduce greatly the affinity of the
corresponding substituted benzenes with extremely nonpolar heptane, making
p X(hep-water) considerably lower than the respective D X for the substituents.
In the octanol–water system, the results indicate that when p X approximates
D X for substitutent X and p Y approximates D Y for substitutent Y in mono-
substituted benzenes, the values of p XX (or p YY) and p XY also approximate the
corresponding values of D XX (or D YY) and D XY for disubstitutents X,X and X,Y
attached to benzene. Supporting data are demonstrated with xylenes and
dichlorobenzenes (p XX D XX) and with toluidines and m-chloroaniline (p XY