Page 80 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 80
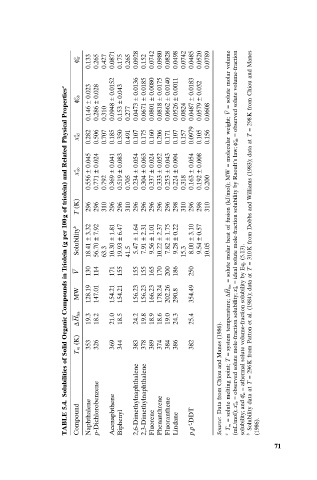
0.133 0.265 0.427 0.175 0.265 0.152 solute molar volume
f° at 0.0871 0.0928 0.0742 0.0980 0.0828 0.0498 0.0742 0.0485 0.0520 0.0789
0.023 0.028 0.0152 0.043 0.0136 0.0185 0.0080 0.0175 0.0140 0.0011 0.0183 0.032 observed solute volume-fraction 298K from Chiou and Manes
Solubilities of Solid Organic Compounds in Triolein (g per 100g of triolein) and Related Physical Properties a
f° ob
0.146 ± 0.286 ± 0.310 0.0948 ± 0.153 ± 0.277 0.0473 ± 0.0671 ± 0.0801 ± 0.0818 ± 0.0662 ± 0.0526 ± 0.0824 0.0487 ± 0.0579 ± 0.0608 – = V
x° id 0.282 0.506 0.707 0.185 0.350 0.491 0.107 0.175 0.160 0.206 0.171 0.107 0.157 0.0979 0.105 0.156 molecular weight; = ob = data at T
0.045 0.024 0.041 0.083 0.054 0.063 0.024 0.052 0.043 0.004 0.054 0.008
0.556 ± 0.771 ± 0.792 0.369 ± 0.519 ± 0.705 0.234 ± 0.304 ± 0.337 ± 0.333 ± 0.253 ± 0.224 ± 0.318 0.163 ± 0.192 ± 0.200
x° ob MW =
(K) 296 296 310 296 296 310 296 296 296 296 296 298 310 296 298 310
T ideal solute mole-fraction solubility by Raoult’s law; f° 310K from Dobbs and Williams (1983);
Solubility b 3.32 18.41 ± 7.92 56.70 ± 63.3 1.81 10.30 ± 6.47 19.93 ± 41.5 1.64 5.47 ± 2.31 7.91 ± 1.01 9.56 ± 2.37 10.22 ± 1.75 7.82 ± 0.22 9.28 ± 15.3 3.10 8.00 ± 0.57 9.54 ± 10.05 solute molar heat of fusion (kJ/mol); (5.13).
130 114 171 155 155 155 165 170 200 186 250 =
V
= = data at T
MW 128.19 147.01 154.21 154.21 156.23 156.23 166.23 178.24 202.26 290.8 354.49 – DH fus ib (1984);
fus
DH 19.3 18.2 21.0 18.5 24.2 19.8 18.9 18.6 19.0 24.3 25.4 system temperature; athermal solute volume-fraction solubility by Eq.
(K) 353 326 369 344 383 378 389 374 384 386 382 observed solute mole-fraction solubility; x° 296K from Patton et al.
T m Data from Chiou and Manes (1986). = T =
5.4. p-Dichlorobenzene 2,6-Dimethylnaphthalene 2,3-Dimethylnaphthalene solute melting point; = ob = at Solubility data at T
TABLE Compound Naphthalene Acenaphthene Biphenyl Fluorene Phenanthrene Fluoranthene Lindane p,p¢-DDT Source: a = T m (mL/mol); x° solubility; and f° b (1986).
71