Page 85 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 85
76 CONTAMINANT PARTITION AND BIOCONCENTRATION
Triolein-Water Partition Coefficient, Log K tw
6
4
2
0
-8 -6 -4 -2
Log (S V)
w
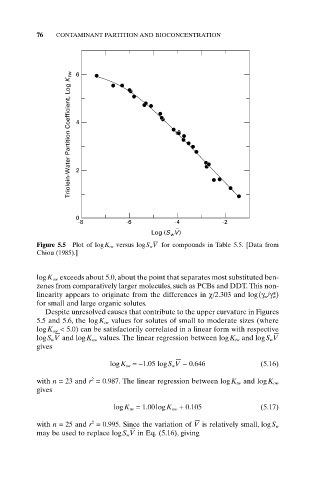
Figure 5.5 Plot of logK tw versus logS wV for compounds in Table 5.5. [Data from
Chiou (1985).]
logK ow exceeds about 5.0, about the point that separates most substituted ben-
zenes from comparatively larger molecules, such as PCBs and DDT. This non-
linearity appears to originate from the differences in c/2.303 and log(g w/g* w)
for small and large organic solutes.
Despite unresolved causes that contribute to the upper curvature in Figures
5.5 and 5.6, the logK tw values for solutes of small to moderate sizes (where
logK ow < 5.0) can be satisfactorily correlated in a linear form with respective
logS w V and logK ow values. The linear regression between logK tw and logS w V
gives
logK tw =-1.05 logS w V - 0.646 (5.16)
2
with n = 23 and r = 0.987. The linear regression between logK tw and logK ow
gives
logK tw = 1.00logK ow + 0.105 (5.17)
2
with n = 25 and r = 0.995. Since the variation of V is relatively small, logS w
may be used to replace logS w V in Eq. (5.16), giving