Page 76 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 76
SUBSTITUENT CONTRIBUTIONS TO PARTITION COEFFICIENTS 67
t - C H
4 9
2 n - C H
3 7
1,3,5 - (CH )
3 3
1,2,4 - Cl 3
1,3 - (CH )
3 2
1,2 - (CH ) 1,3 - Cl 2
3 2
CF 3 1,2 - Cl 2
CH = CH 2 l C 2 H 5
Br
Cl
CH 3
F
0
OCH 3
x (heptane-water) COCH 3 NO 2
CHO
π CN
1-NH -3-Cl
2
1-NH -3-CH 3
2
-2 1-NH 2 -2-CH 3
NH 2
COOH
OH
CH COOH
2
-4
-2 0 2
∆ x
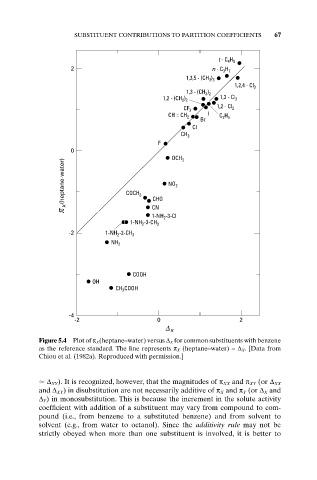
Figure 5.4 Plot of p X(heptane–water) versus D X for common substituents with benzene
as the reference standard. The line represents p X (heptane–water) =D X . [Data from
Chiou et al. (1982a). Reproduced with permission.]
D XY). It is recognized, however, that the magnitudes of p XX and p XY (or D XX
and D XY) in disubstitution are not necessarily additive of p X and p Y (or D X and
D Y) in monosubstitution. This is because the increment in the solute activity
coefficient with addition of a substituent may vary from compound to com-
pound (i.e., from benzene to a substituted benzene) and from solvent to
solvent (e.g., from water to octanol). Since the additivity rule may not be
strictly obeyed when more than one substituent is involved, it is better to